- Title
-
Targeting Radiation Resistance in Oesophageal Adenocarcinoma with Pyrazinib-Functionalised Gold Nanoparticles
- Authors
- Marcone, S., Spadavecchia, J., Khan, M., Vella, G., O'Connell, F., Pendino, M., Menon, M., Donohoe, C., Narayanasamy, R., Reynolds, J.V., Maher, S.G., Lynam-Lennon, N., Kennedy, B., Prina-Mello, A., O'Sullivan, J.
- Source
- Full text @ Cancers
|
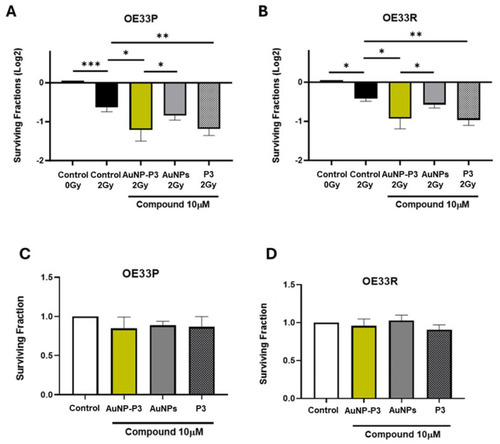
Pyrazinib-functionalised gold nanoparticles (AuNP-P3) enhanced radiosensitivity in an the OAC cell line model of radioresistance. The effect of pyrazinib-coupled gold nanoparticles (AuNP-P3), pyrazinib (P3), and gold nanoparticles (AuNPs) at 10 µM treatment on the radiosensitivity of OE33P (radiation sensitive) and OE33R (radioresistant) cells was assessed by clonogenic assays and compared to the control (0.1% DMSO + 0.1% water). Surviving fraction of OE33P ( |
|
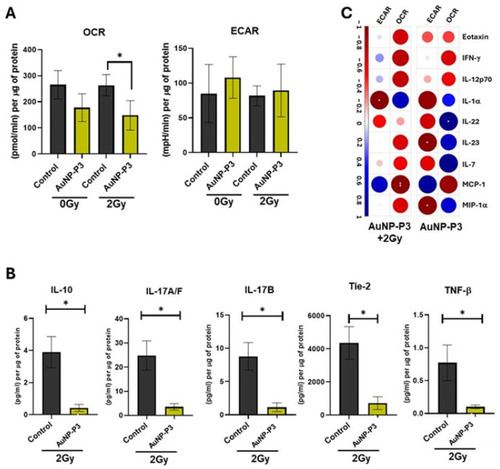
Pyrazinib-functionalised gold nanoparticles (AuNP-P3) treatment reduced oxidative phosphorylation and glycolysis in response to ionizing radiation in an OAC cell line model of radioresistance. OE33P (radiation sensitive cells) and OE33R (radiation resistant cells) were treated for 24 h with the compounds at 10 µM or with the control (0.1% DMSO + 0.1% water) and subsequently irradiated at 2 Gy X-ray radiation. After 24 h, cells were used to measure metabolic rates and the supernatants were stored for multiplex ELISA. Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured in real-time using Seahorse Biosciences XFe24 analyser. Comparison of basal and radiation-induced OCR ( |
|
Pyrazinib-functionalised gold nanoparticles (AuNP-P3) significantly altered the levels of secreted mediators in response to ionizing radiation in an OAC cell line model of radioresistance. The secreted levels of protein in OE33P (radiation sensitive) and OE33R (radiation resistant) cells were evaluated by 54-plex ELISA. Here, we report data only for the significantly modulated proteins. ( |
|
Pyrazinib-functionalised gold nanoparticles (AuNP-P3) decreased metabolic parameters and released mediators in OAC tumour explants in response to ionizing radiation. OAC explants were treated with pyrazinib-functionalised gold nanoparticles (AuNP-P3) at 10 µM, and after 18 h incubation, explants were exposed to 2 Gy X-ray radiation. After 6h incubation, OCR and ECAR were measured in real-time. Tissue-conditioned media were collected after Seahorse measurement and used for multiplex ELISA analysis. ( |
|
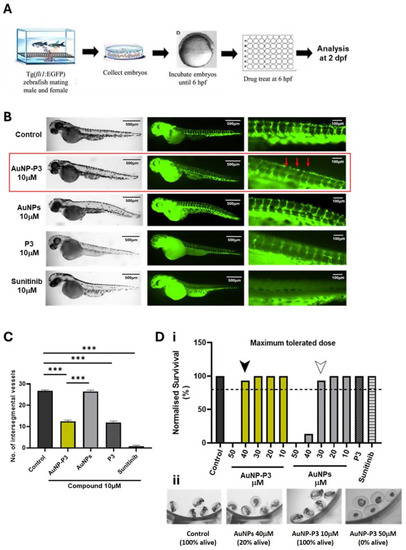
Pyrazinib-functionalised gold nanoparticles (AuNP-P3) inhibited developmental angiogenesis in Tg(fli1:EGFP) zebrafish. ( |