- Title
-
CM082 suppresses hypoxia-induced retinal neovascularization in larval zebrafish
- Authors
- Zhang, J.L., Fan, D.G., Yin, W., Hu, B.
- Source
- Full text @ Front Pharmacol
|
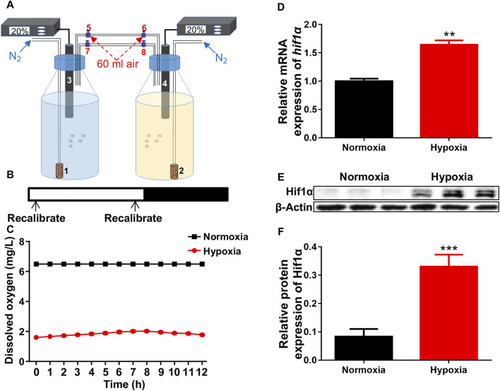
Hypoxic setup for larval zebrafish. |
|
CM082 suppressed retinal neovascularization induced by hypoxia. |
|
CM082 rescued cell loss in the area of GCL induced by hypoxia. |
|
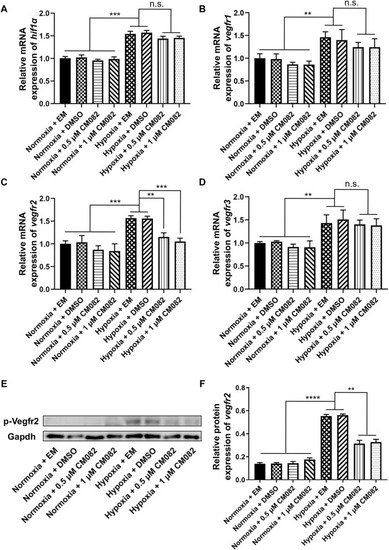
CM082 inhibited the expression of |
|
CM082 rescued optokinetic response deficiency induced by hypoxia. |
|
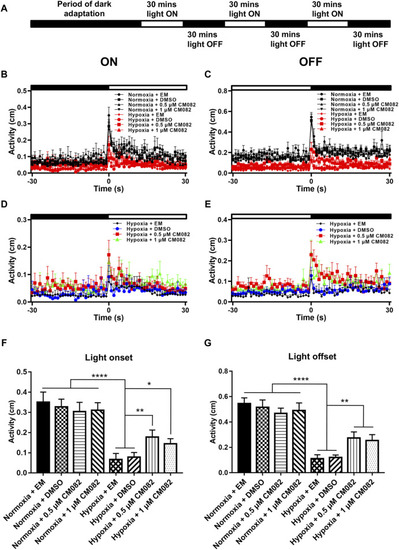
CM082 partially rescued visual motor response (VMR) deficiency induced by hypoxia. |