- Title
-
Context-dependent hyperactivity in syngap1a and syngap1b zebrafish models of SYNGAP1-related disorder
- Authors
- Sumathipala, S.H., Khan, S., Kozol, R.A., Araki, Y., Syed, S., Huganir, R.L., Dallman, J.E.
- Source
- Full text @ Front. Mol. Neurosci.
|
Zebrafish |
|
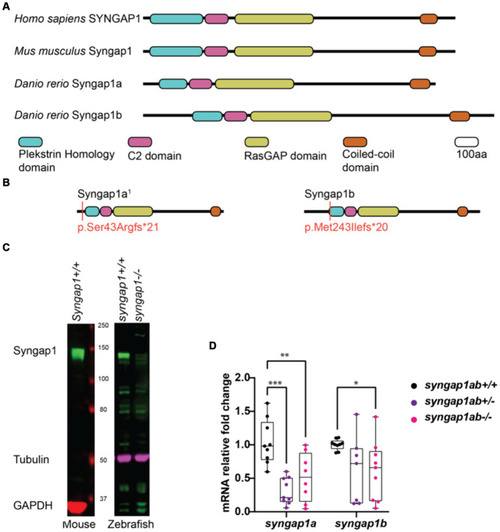
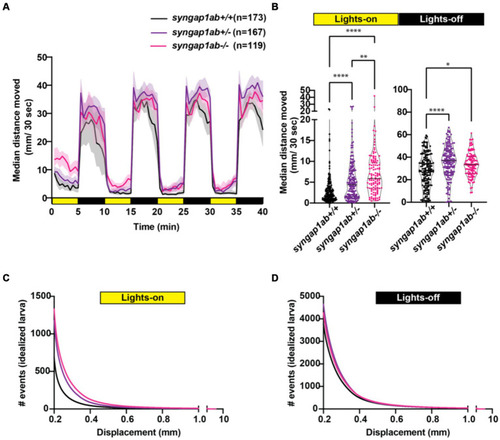
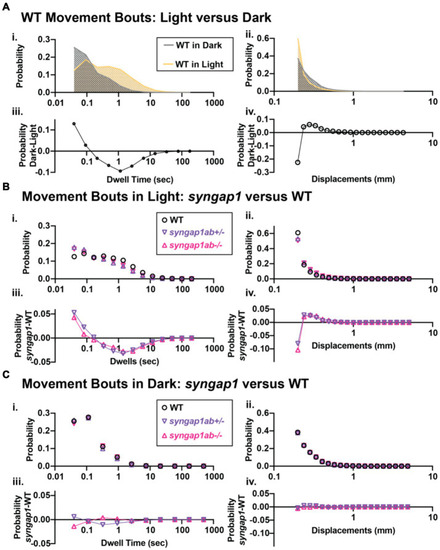
Zebrafish loss-of-function model for human SYNGAP1-RD. |
|
|
|
|
|
|