- Title
-
Zebrafish mylipb attenuates antiviral innate immunity through two synergistic mechanisms targeting transcription factor irf3
- Authors
- Li, Z., Li, J., Li, Z., Song, Y., Wang, Y., Wang, C., Yuan, L., Xiao, W., Wang, J.
- Source
- Full text @ PLoS Pathog.
|
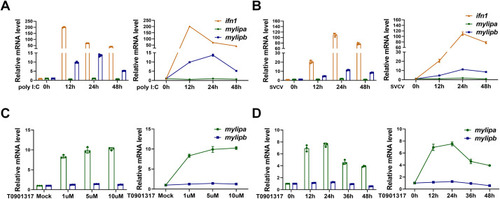
Zebrafish (A) Zebrafish |
|
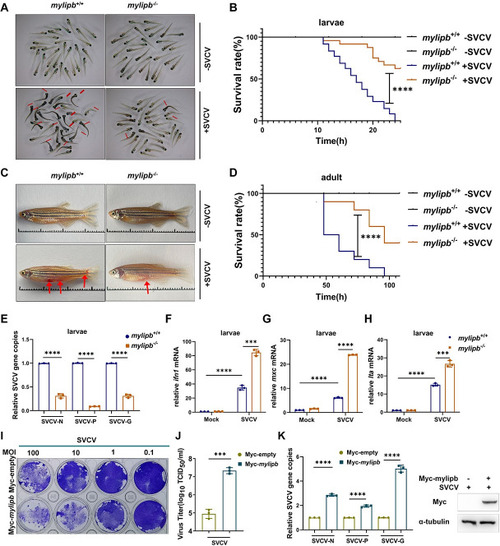
Zebrafish (A) Representative images of |
|
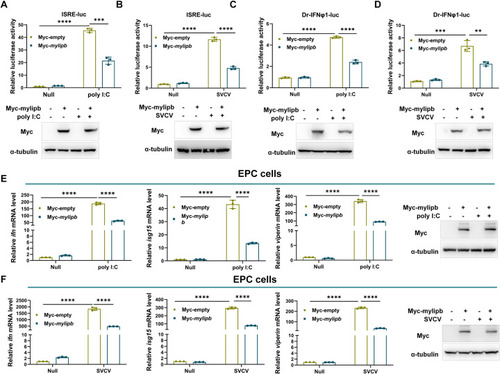
Zebrafish (A-B) Overexpression of |
|
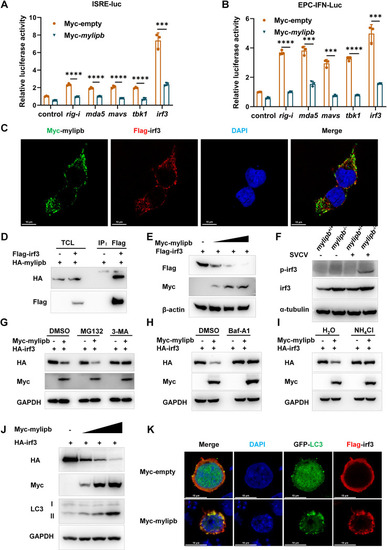
Mylipb interacts with irf3 and induces autophagic degradation of irf3. (A) Overexpression of |
|
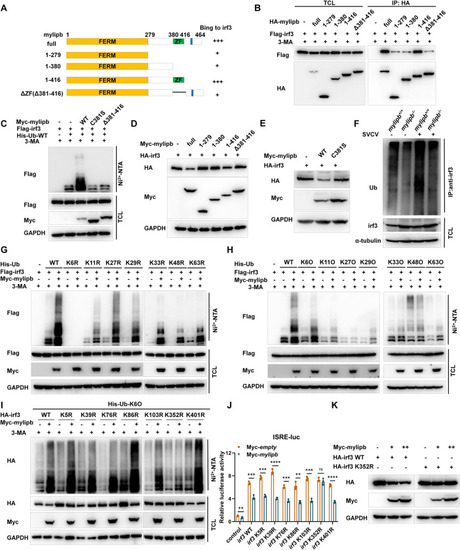
Mylipb negatively regulates irf3 by promoting K6-linkd ubquitination of irf3 at lysine 352. (A-B) The interaction between mylipb and irf3 protein mostly depended on the ZF domain of mylipb protein. HEK293T cells seeded in 100-mm dishes were transfected with the indicated plasmids (4 μg each). After 24 h, the cells were treated with 3-MA for 8 h. Total cell lysates were immunoprecipitated (IP) with anti-HA antibody conjugated agarose beads. Then, the immunoprecipitates and cell lysates were detected with anti-HA or anti-Flag Ab, respectively. (C) Mylipb promoted irf3 ubiquitination depended on ZF domain and ubiquitin ligase activity of mylipb. HEK 293T cells were transfected with Flag- |
|
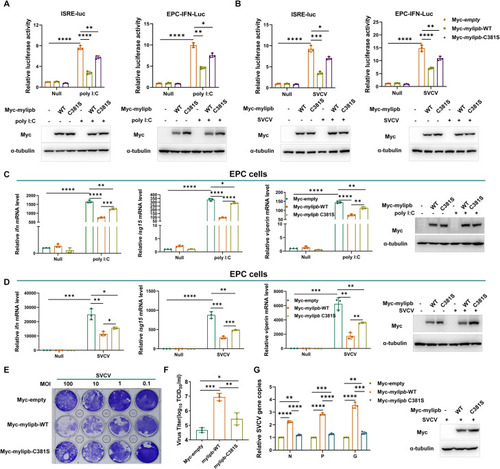
Mylipb negatively regulates cellular antiviral response mainly dependent of its ubiquitin ligase activity. (A) ISRE reporter activity and EPC-IFN-Luc activity in Myc-empty vector or Myc- |
|
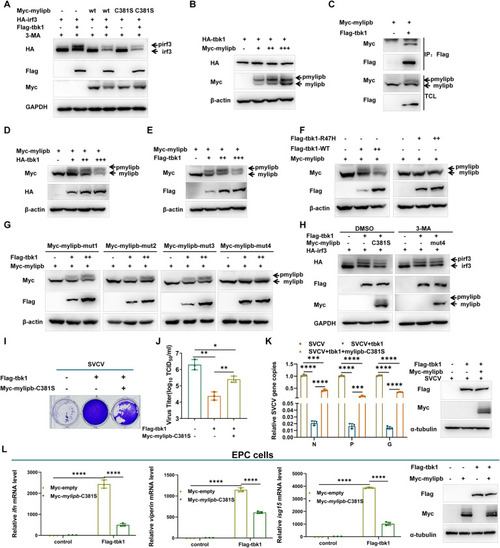
Mylipb decreases tbk1-mediated irf3 phosphorylation and cellular antiviral response. (A) Overexpression of |
|
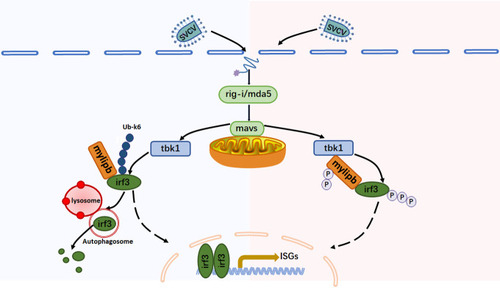
A model on the two synergistic mechanisms of zebrafish mylipb in negative regulation of antiviral innate immunity. Upon viral infection, zebrafish rig-i/mda5 recognizes the viral RNA and interacts with mavs, leading to the recruitment of tbk1, which phosphorylates irf3 and then induces IFN production. Zebrafish mylipb, an E3 ubiquitin ligase, increases K6-linked polyubiquitination at Lys 352, leading to the induction of autophagic degradation of irf3, while at the same time reducing irf3 phosphorylation by acting as a substrate for tbk1, thereby preventing the expression of IFN. |