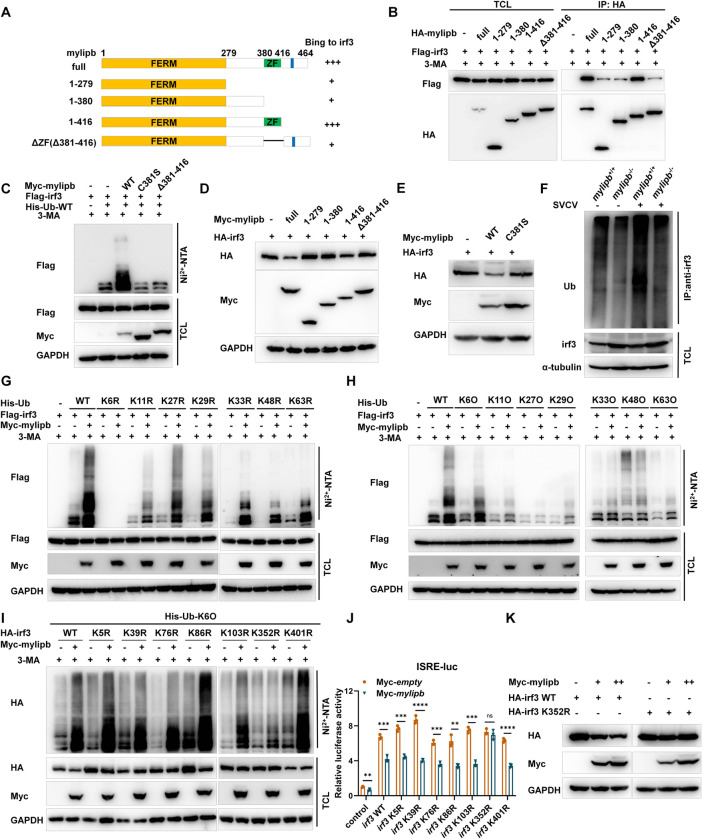
Fig 5 Mylipb negatively regulates irf3 by promoting K6-linkd ubquitination of irf3 at lysine 352.
(A-B) The interaction between mylipb and irf3 protein mostly depended on the ZF domain of mylipb protein. HEK293T cells seeded in 100-mm dishes were transfected with the indicated plasmids (4 μg each). After 24 h, the cells were treated with 3-MA for 8 h. Total cell lysates were immunoprecipitated (IP) with anti-HA antibody conjugated agarose beads. Then, the immunoprecipitates and cell lysates were detected with anti-HA or anti-Flag Ab, respectively. (C) Mylipb promoted irf3 ubiquitination depended on ZF domain and ubiquitin ligase activity of mylipb. HEK 293T cells were transfected with Flag-