- Title
-
Macrophage depletion overcomes human hematopoietic cell engraftment failure in zebrafish embryo
- Authors
- El Omar, R., Abdellaoui, N., Coulibaly, S.T., Fontenille, L., Lanza, F., Gachet, C., Freund, J.N., Negroni, M., Kissa, K., Tavian, M.
- Source
- Full text @ Cell Death Dis.
|
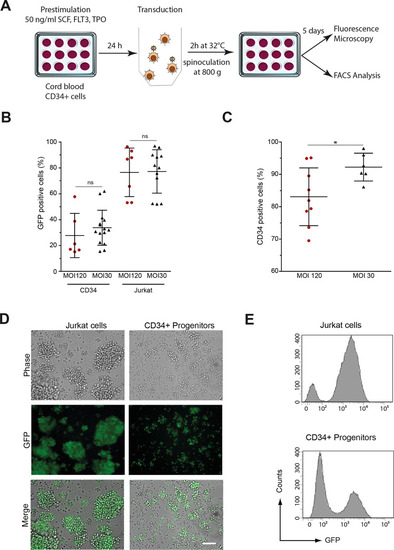
Transgene transfer and GFP expression after transduction of human CD34+ and Jurkat cells. |
|
Transplantation of human JK-GFP cells in zebrafish embryos, colonization of hematopoietic organs and proliferation. |
|
Human JK-GFP cells phagocytosis by zebrafish embryonic macrophages. |
|
CD34-GFP cells phagocytosis by zebrafish embryonic macrophages. |
|
Comparison of injection sites in the zebrafish embryo. |
|
Genetic and chemical depletion of macrophages in zebrafish embryos. Primitive macrophages were genetically and chemically depleted before cell engraftment in Tg ( |