- Title
-
Lapatinib combined with doxorubicin causes dose-dependent cardiotoxicity partially through activating the p38MAPK signaling pathway in zebrafish embryos
- Authors
- Du, K., Liu, Y., Zhang, L., Peng, L., Dong, W., Jiang, Y., Niu, M., Sun, Y., Wu, C., Niu, Y., Ding, Y.
- Source
- Full text @ Biomed. Pharmacother.
|
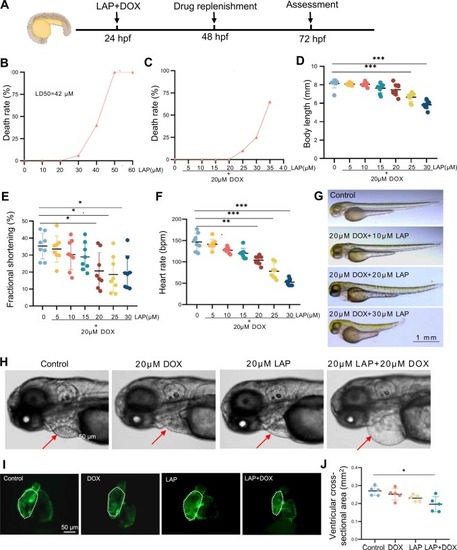
Establishment of a dose-dependent cardiotoxicity model induced by lapatinib (LAP) combined with doxorubicin (DOX) in zebrafish embryos. (A) Experimental scheme showing combined LAP and DOX exposure to induce cardiotoxicity in zebrafish embryos. hpf: hours postfertilization. (B) Mortality of zebrafish embryos exposed to different doses of LAP alone. (C-D) Mortality (C) and body length (D) of zebrafish embryos exposed to different doses of LAP combined with 20 µM DOX. N=9, one-way analysis of variance (ANOVA). (E-F) Quantification of fractional shortening (FS) (E) and heart rate (F) in zebrafish embryos exposed to different doses of LAP combined with 20 µM DOX. bpm, beats per minute. N= 8, one-way ANOVA. (G-H) Representative images of embryonic morphology at 72 hpf after exposure to indicated doses of LAP and/or 20 µM DOX. The arrows point to the pericardium. (I-J) Representative fluorescence images of embryonic hearts (I) and quantification of the ventricular cross-sectional areas (J) of the Tg(cmlc2:EGFP) transgenic fish at 72 hpf after 20 µM LAP and 20 µM DOX combinational exposure compared to 20 µM LAP or 20 µM DOX alone or the DMSO control. N=5, one-way ANOVA; *P<0.05, **P<0.01, ***P<0.001. |
|
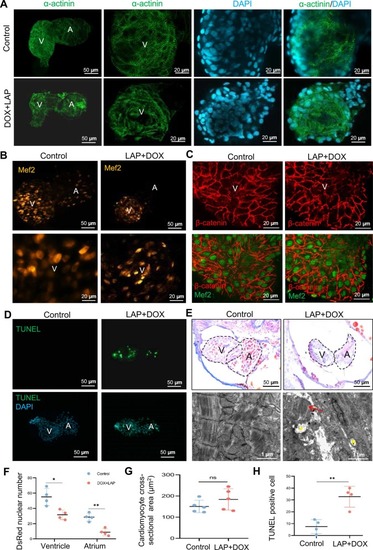
Characterization of the combined LAP and DOX exposure-induced cardiotoxicity phenotype in zebrafish embryos. (A) Representative fluorescence images of embryonic hearts immune-stained with the anti-α-actinin antibody in the combined LAP and DOX exposure-induced cardiotoxicity model and the DMSO control. A, atrium; V, ventricle. (B) Representative fluorescence images of embryonic hearts in the Tg(cmlc2:DsRed) transgenic fish after combined LAP and DOX exposure compared to the DMSO control at 72 hpf. (C) Representative images outlining cross-sectional area of cardiomyocytes by immunostaining with anti–β-catenin (red) and anti–myocyte enhancer factor-2 (Mef2, green) antibodies in the LAP and DOX combination-induced cardiotoxicity model and DMSO control at 72 hpf. (D) Representative fluorescence images of TUNEL-stained embryonic hearts in the LAP and DOX combination-induced cardiotoxicity model and DMSO control. (E) Upper panels, shown are Masson’s trichrome stain images to detect fibrosis from the heart chambers of LAP and DOX treated embryos compared to DMSO control at 72 hpf. Lower panels, transmission electron microscopy (TEM) images confirmed the disruption of sarcomere structure and empty mitochondrial defects in the combined LAP and DOX exposure-induced cardiotoxicity model. The arrow indicates degenerative sarcomeres. The asterisks point to empty mitochondrial defects. (F-H) Quantification of cardiomyocyte numbers (F), cardiomyocyte cross-section area (G) and the TUNEL index (H) in the LAP and DOX combination-induced cardiotoxicity model and DMSO control at 72 hpf. N=4–5, Student’s t test. *P<0.05,**P<0.01. A, atrium; V, ventricle. |
|
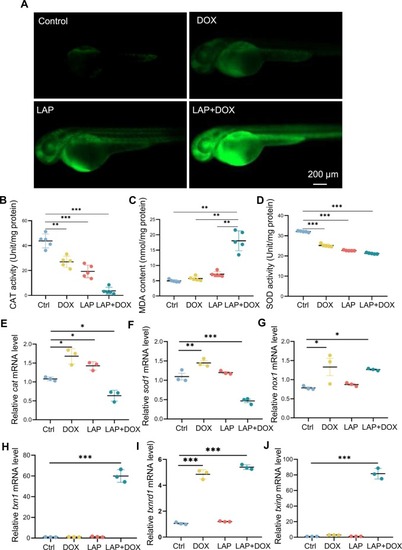
Oxidative stress was increased in the combined LAP and DOX exposure-induced cardiotoxicity model. (A) Representative fluorescence images of ROS staining in the combined LAP and DOX exposure-induced cardiotoxicity model compared to embryos exposed to LAP or DOX alone or DMSO-treated control embryos. (B-D) Quantification of other ROS-related indices, including catalase (CAT) (B), superoxide dismutase (SOD) (C) and malondialdehyde (MDA) (D), in the LAP and DOX combination-induced cardiotoxicity model compared to embryos exposed to LAP or DOX alone or DMSO-treated control embryos. N=5, one-way ANOVA; (E-J) Quantitative RT-PCR analysis of ROS-related gene expression in the LAP and DOX combination-induced cardiotoxicity model compared to embryos exposed to LAP or DOX alone or DMSO-treated control embryos. N=3 biological repeats. one-way ANOVA. *P<0.05, **P<0.01, ***P<0.001. |
|
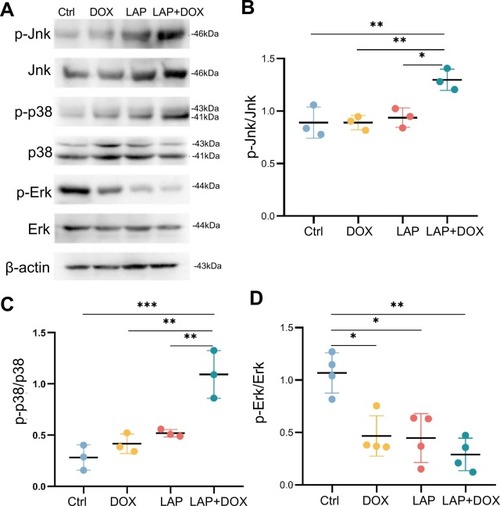
The MAPK signaling pathway was dysregulated in the combined LAP and DOX exposure-induced cardiotoxicity model. (A-D) Western blotting (A) and quantification of MAPK downstream target proteins, including phosphorylated Jnk (p-Jnk) normalized to total Jnk (B), phosphorylated p38 (p-p38) normalized to total p38 (C) and phosphorylated Erk (p-Erk) normalized to total Erk (D), in the combined LAP and DOX exposure-induced cardiotoxicity model compared to embryos exposed to LAP or DOX alone or DMSO-treated control embryos. N=3–4 biological replicates, one-way ANOVA; *P<0.05, **P<0.01, ***P<0.001. |
|
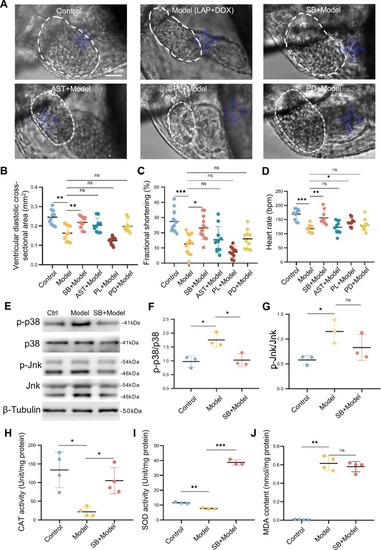
SB203580 alleviated combined LAP and DOX exposure-induced cardiotoxicity. (A-D) Representative bright field images (A) and quantification of ventricular cross-sectional areas (B), the percent fractional shortening (FS) (C), and heart rates (D) of embryos in the LAP and DOX combination-induced cardiotoxicity model that were treated with small molecular inhibitors of oxidative stress or the MAPK signaling pathway. The ventricle borders are outlined by dashed white lines.N=10; one-way ANOVA. SB, SB203580; AST, Astaxanthin; PL, Piperlongumine; PD, PD0325901. (E-G) Western blotting (E) and quantification of p-p38 normalized to total p38 and p-Jnk normalized to total Jnk (G) in the combined LAP and DOX exposure-induced cardiotoxicity model that was treated with 5 μM SB203580 or the DMSO control. N=3 biological replicates, one-way ANOVA. (H-J) Quantification of ROS-related indices, including CAT (H), SOD (I) and MDA (J), in the LAP and DOX combination-induced cardiotoxicity model that was treated with 5 μM SB203580 or the DMSO control. N=4, one-way ANOVA; *P<0.05, **P<0.01, ***P<0.001, ns, not significant. |
|
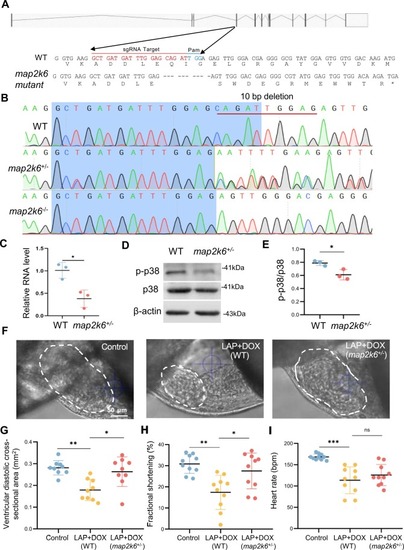
The map2k6 frameshift mutation reduced combined LAP and DOX exposure-induced cardiotoxicity. (A) Schematic of the 10-nucleotide deletion allele generated by the injection of a single guide RNA (sgRNA) targeting the sequence within the 4th exon of the map2k6 gene. Dashed lines indicate deleted nucleotides. The asterisk indicates the predicted early translational stop codon. (B) Chromographs illustrating the sequences of WT and the map2k6 mutant allele with the 10-nucleotide deletion in F1 heterozygous and homozygous fish. (C) Quantitative RT-PCR demonstrated transcription reduction in the map2k6 heterozygous mutant. N=3 biological replicates. (D-E) Western blotting (D) and quantification (E) of p-p38 protein normalized by total p38 in map2k6 heterozygous mutant fish compared to that in WT Control hearts. N=3, Student’s t test. (F-I) Representative images (F) and quantification of the ventricular diastolic cross-sectional area (outlined by dashed white lines) (G), the percent fractional shortening (FS) (H) and heart rates (I) of the map2k6 heterozygous mutant embryos compared to WT embryos after exposure to LAP plus DOX or the DMSO control at 72 hpf. N=9–10, one-way ANOVA. *P<0.05, **P<0.01, ***P<0.001, ns, not significant; Scale bar: 50 µm. |
|
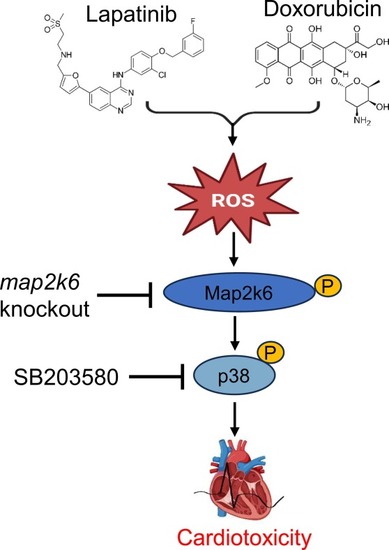
LAP combined with DOX exposure causes cardiotoxicity by activating the p38MAPK signaling pathway. |