- Title
-
Inversely Regulated Inflammation-Related Processes Mediate Anxiety-Obesity Links in Zebrafish Larvae and Adults
- Authors
- Yehuda, H., Madrer, N., Goldberg, D., Soreq, H., Meerson, A.
- Source
- Full text @ Cells
|
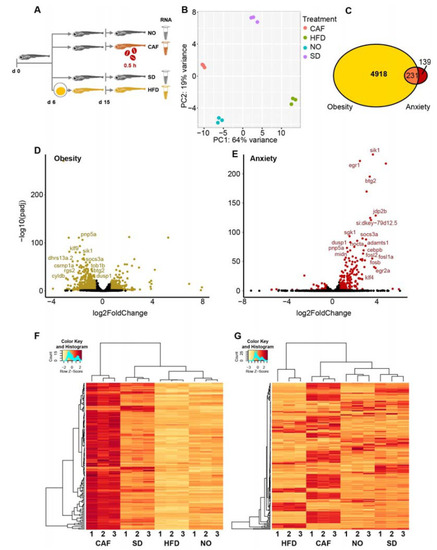
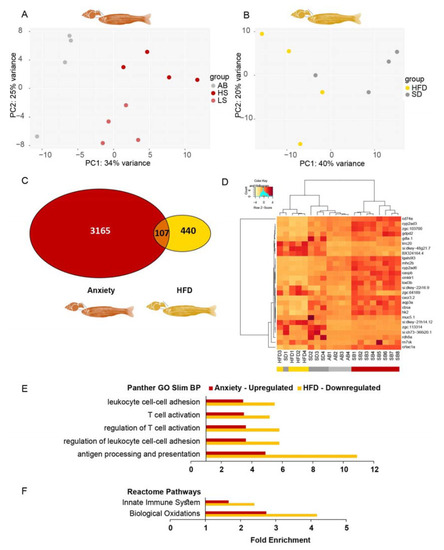
Most anxiety-upregulated larvae transcripts were downregulated in obesity. ( |
|
Larval inflammation/immune pathways are upregulated in anxiety and downregulated in obesity. ( |
|
Most differentially expressed (DE) long intergenic noncoding RNAs (lincRNAs) were upregulated in the anxiety model and downregulated in the obesity model, similar to protein-coding genes. ( |
|
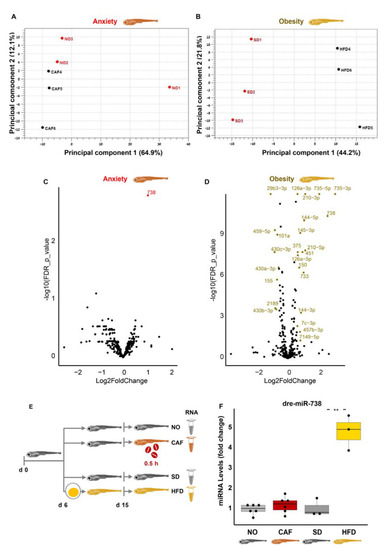
Null involvement of microRNAs (miRNAs) in the anxiety and obesity link in zebrafish larvae. ( |
|
Transfer RNA fragments (tRFs), as well, are inversely regulated in anxiety and obesity. ( |
|
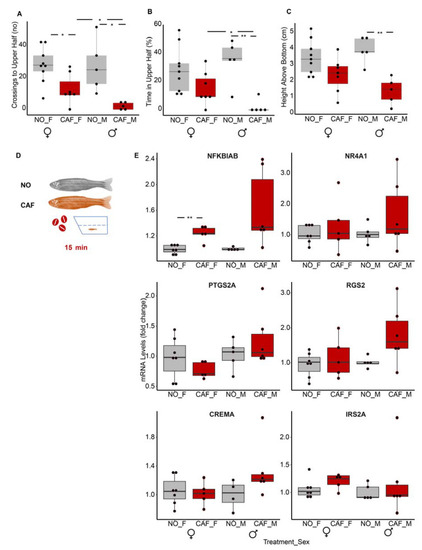
Obese larvae failed to present anxiety-like behavior and vice versa. ( |
|
The nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) inhibitor (nfkbiab) transcript was upregulated in whole bodies of anxiety-induced middle-aged adult males and females. Anxiety-like behavioral tests: ( |
|
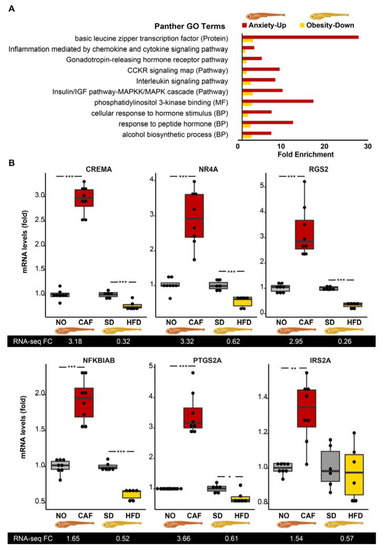
Immune system-related gene ontology (GO) terms are enriched among transcripts increased in anxiety and decreased under high-fat diet (HFD) conditions in adult zebrafish. ( |