- Title
-
Autism-associated gene shank3 is necessary for social contagion in zebrafish
- Authors
- Kareklas, K., Teles, M.C., Dreosti, E., Oliveira, R.F.
- Source
- Full text @ Mol Autism
|
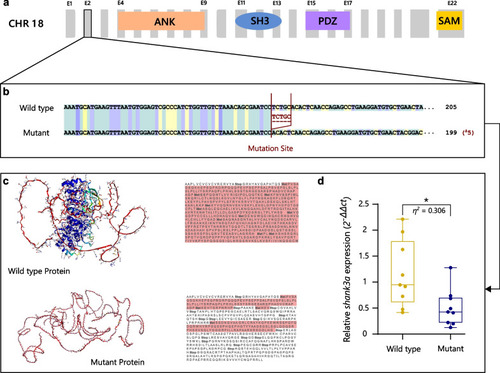
Characterization of the genetic mutation of |
|
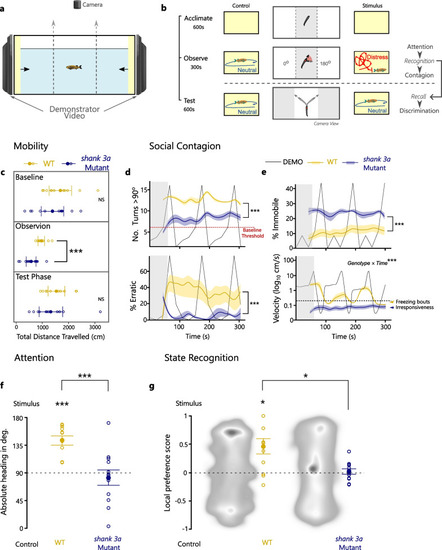
Experimental assessment of PHENOTYPE:
|
|
Quantification of changes in genetic neuroplasticity markers derived by the |