- Title
-
Proteinase 3 promotes formation of multinucleated giant cells and granuloma-like structures in patients with granulomatosis with polyangiitis
- Authors
- Henderson, S.R., Horsley, H., Frankel, P., Khosravi, M., Goble, T., Carter, S., Antonelou, M., Evans, R.D.R., Zhang, X., Chu, T.Y., Lin, H.H., Gordon, S., Salama, A.D.
- Source
- Full text @ Ann. Rheum. Dis.
|
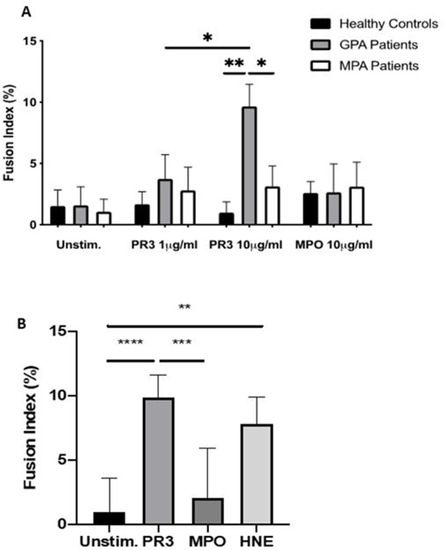
PR3 induces MGC formation in monocytes from patients with GPA. Monocytes from patients with GPA, patients with MPA and healthy controls were stimulated with PR3 and MPO (n=10) (A). There was a dose–response effect seen in patients with GPA with increasing MGC formation at PR3 10 µg/mL compared with patients with MPA and healthy controls. MPO did not stimulate MGC formation. HNE was also tested (B) and showed a similar increase in MGC formation in patients with GPA. Values plotted as median and 95% CI; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Difference between conditions determined by one-way and two-way ANOVA tests. ANOVA, analysis of variance; GPA, granulomatosis with polyangiitis; HNE, human neutrophil elastase; MGC, multinucleated giant cells; MPA, microscopic polyangiitis; MPO, myeloperoxidase; PR3, proteinase 3; unstim, unstimulated. |
|
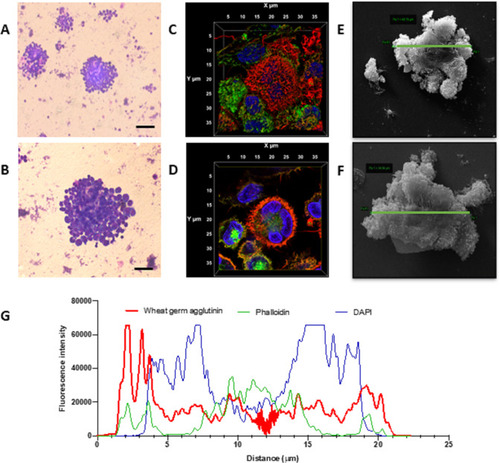
Monocyte fusion is stimulated by PR3. Monocytes stimulated with PR3 were first imaged by Giemsa staining. Distinct cellular aggregates formed in the presence of PR3 (A). At higher magnification (B), a larger cellular aggregate is present showing dense nuclear staining and cytoplasmic fusion (scale bars 100 µm). Three-dimensional volume rendering of an aggregate, including all acquired slices by confocal microscopy, was undertaken using cell membrane (red), cytoskeletal (green) and nuclear staining (blue) (C, D). Midslice (D) confirmed membrane fusion, cytoskeletal rearrangement and a single cellular structure with fusion of three monocytes, defining an MGC. Scanning electron microscopy highlighted monocyte fusion, with representative images showing an MGC size of 48.8 µm (E) and 34.6 µm (F). Membrane fusion was measured by profile plot of the fluorescence intensity of the three channels over the x/y diameter (G). There was prominent membrane staining encasing cytoskeleton and three nuclei. MGC, multinucleated giant cells; PR3, proteinase 3. |
|
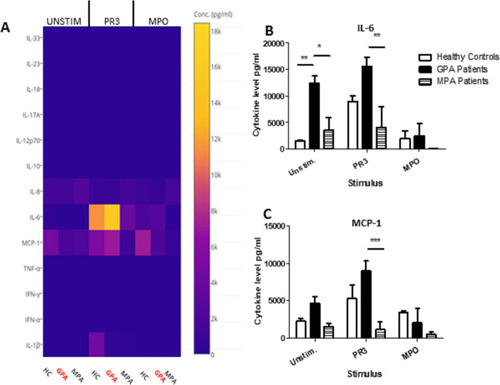
PR3-induced MGC stimulates proinflammatory cytokines. Cytokines and chemokines were measured in MGC cell culture supernatants in unstimulated, PR3-stimulated or MPO-stimulated cells (A). IL-6 production increased in the presence of PR3 in patients with GPA compared with patients with MPA, but was not significantly different from HC (B). A reduction in IL-6 production was seen in patients with GPA following stimulation with MPO. Monocyte Chemoattractant protein (MCP)-1 production (C) was also increased in patients with GPA following stimulation with PR3 compared with patients with MPA. MPO stimulation did not have any effect on differences in IL-6 or MCP-1 production. Values plotted as median and IQR; *p<0.05, **p<0.01, ***p<0.001. Difference between multiple conditions determined by two-way ANOVA. ANOVA, analysis of variance; GPA, granulomatosis with polyangiitis; HC, healthy controls; IL, interleukin; IFN, Interferon; MGC, multinucleated giant cells; MPA, microscopic polyangiitis; MPO, myeloperoxidase; PR3, proteinase 3; TNF, tumour necrosis factor. |
|
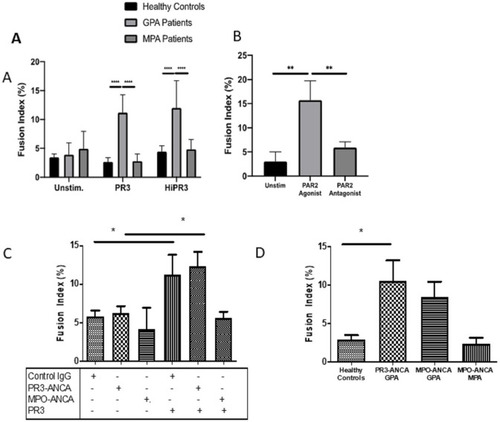
MGC forms in the presence of enzymatically active and inactive PR3. No statistical difference in fusion index was seen in the cells from patients with GPA when stimulated with enzymatically active or heat-inactivated (Hi) PR3 (A). PAR-2 agonism increased the fusion index in patients with GPA, and PAR-2 antagonism significantly inhibited the rates of MGC formation (B). The presence of PR3-ANCA or MPO-ANCA alone had no effect on fusion index compared with control immunoglobulin (C); however, when cultured with PR3, there was a significant increase in MGC formation in the presence of PR3-ANCA and control immunoglobulin compared with MPO-ANCA. Patients with GPA with different ANCA subtypes (D). Patients with PR3-ANCA GPA showed a significant increase in monocyte fusion compared with healthy controls and patients with MPO-ANCA MPA; however, a similar increase in MGC formation was seen in patients with MPO-ANCA GPA. Values plotted as median and 95% CI; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Difference between conditions determined by one-way and two-way ANOVA tests. ANCA, antineutrophil cytoplasm antibodies; ANOVA, analysis of variance; GPA, granulomatosis with polyangiitis; MGC, multinucleated giant cells; MPA, microscopic polyangiitis; MPO, myeloperoxidase; PAR-2, protease-activated receptor-2; PR3, proteinase 3; unstim, unstimulated. |
|
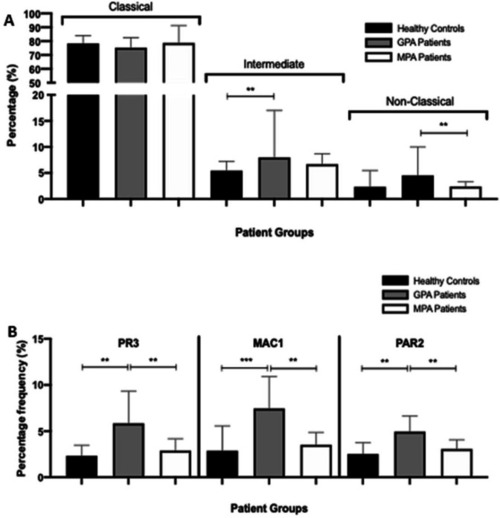
PR3, MAC-1 and PAR-2 expression is increased on the monocytes from patients with GPA. Following staining, the proportions of classic (CD14hi CD16lo), intermediate (CD14hi CD16hi) and non-classic (CD14lo CD16hi) monocytes were calculated. There was a significant increase in intermediate monocytes in patients with GPA compared with healthy controls and non-classic monocytes in patients with GPA compared with patients with MPA (A). PR3, MAC-1 and PAR-2 expression on the monocytes of patients with CD14-positive GPA was significantly increased compared with healthy controls and patients with MPA (B). Values plotted as median and 95% CI; *p<0.05, **p<0.01, ***p<0.001. Difference between conditions determined by one-way and two-way ANOVA tests. ANOVA, analysis of variance; GPA, granulomatosis with polyangiitis; MAC-1, macrophage -1 antigen; MPA, microscopic polyangiitis; PAR-2, protease-activated receptor-2; PR3, proteinase 3. |
|
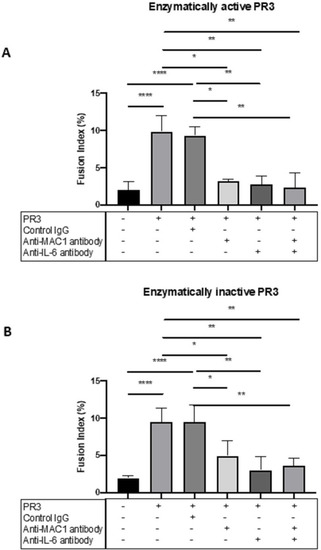
MGC formation is dependent on MAC-1, PAR-2 and IL-6. The effect of MAC-1 and IL-6 inhibition was tested in patients with GPA by culturing monocytes with enzymatically active or inactive PR3 and measuring MGC formation in the presence or absence of anti-MAC-1 antibody, anti-IL-6 antibody or control immunoglobulin. Consistently, PR3 stimulated an increase in MGC formation as well as in the presence of control immunoglobulin with enzymatically active and inactive PR3 (A, B). Anti-MAC-1 antibody and anti-IL-6 antibody reduced MGC formation in both conditions, and the effect of combined anti-MAC-1 antibody and anti-IL-6 antibody also significantly reduced MGC formation. Values plotted as median and 95% CI; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Difference between conditions determined by one-way ANOVA tests. ANOVA, analysis of variance; IL, interleukin; MGC, multinucleated giant cells; PAR-2, protease-activated receptor-2; PR3, proteinase 3. |
|
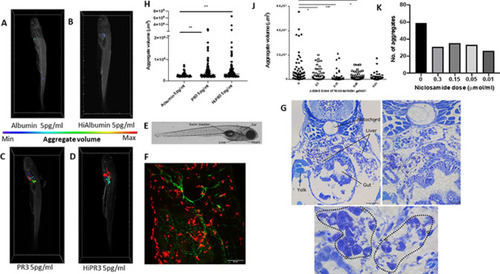
Enzymatically active and inactive PR3 stimulates macrophage aggregation and MGC formation in zebrafish and is inhibited by niclosamide. MPEg-cherry zebrafish (n=10 per group) were injected with human PR3 or albumin (intact or following heat inactivation) and imaged by lightsheet microscopy. Aggregate volumes were calculated by three-dimensional rendering of images following application of automated thresholds. Colour scale was used to demonstrate minimal and maximal aggregate volumes. Albumin with or without heat inactivation (A, B) showed minimal macrophage aggregation compared with enzymatically active and inactive PR3 at 5 pg/mL (C, D). Scale bar 100 µm. (F) Maximum projection of a 5-day-old transgenic larva (tg(flt4:mCitrine, mpeg::mCherry)) injected with PR3 into the yolk sac at 24 hours post fertilisation, at the level shown in E. The developing lymphatic system is labelled in green and peripheral macrophages are labelled in red. (E) Brightfield micrograph of a 5-day-old transgenic zebrafish larva injected with PR3 at 1 day post fertilisation, with certain anatomical structures labelled. Dashed line represents the approximate location of sections shown in G. (G) Plastic sections through the same zebrafish larva at multiple levels. MGCs can be seen (highlighted by white stars) and loose granulomas are outlined (dotted lines). Scale bars are 100 µm, 20 µm and 10 µm, respectively. Counting the number and calculating the size of the macrophage aggregates showed a significant increase in both the number and volume of macrophage aggregates in MPEg-cherry zebrafish injected with enzymatically active or inactive PR3 compared with albumin (H). Niclosamide was administered to zebrafish water following injection with PR3. Compared with PR3 alone (J, K), there was a reduction in aggregate volume and number in zebrafish injected with 0.3 µmol/mL, 0.15 µmol/mL and 0.05 µmol/mL niclosamide. Values plotted as median and 95% CI; *p<0.05, **p<0.01, ***p<0.001. Difference between conditions determined by two-way ANOVA. ANOVA, analysis of variance; Hi, heat-inactivated; MGC, multinucleated giant cells; MPeg, macrophage expressed; PR3, proteinase 3. |