- Title
-
Iron supplementation inhibits hypoxia-induced mitochondrial damage and protects zebrafish liver cells from death
- Authors
- Hu, R., Li, G., Xu, Q., Chen, L.
- Source
- Full text @ Front. Physiol.
|
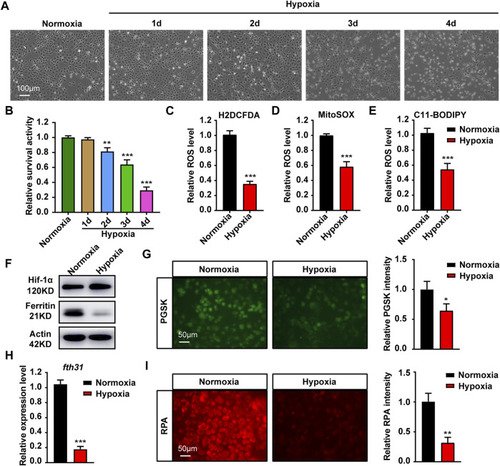
Hypoxia stress affects cell growth, ROS production and iron metabolism in cytoplasma and mitochondria. |
|
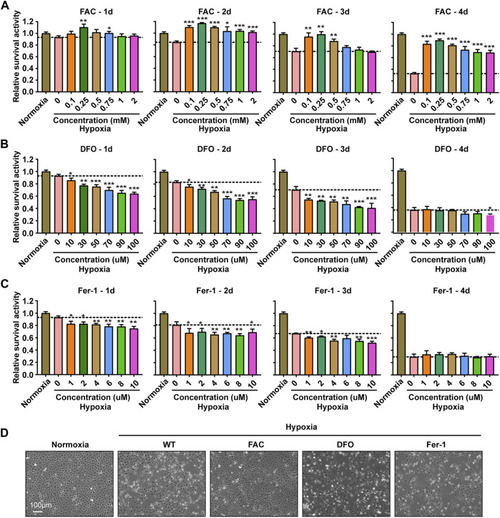
Iron supplementation could improve the survival activity of ZFL cells under hypoxia stress, but iron chelation and inhibition of ferroptosis could not. |
|
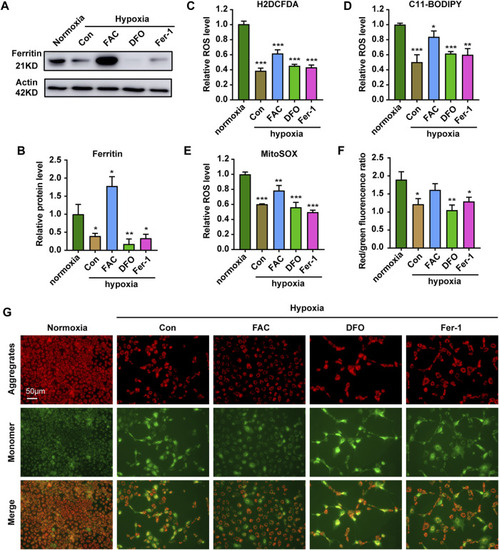
Iron supplementation can increase ROS level and reduce mitochondrial damage in cells under hypoxia stress. |
|
RNA-seq analysis revealed significant transecriptomic changes in hypoxia ZFL cell. |
|
Iron supplementation increases the proportion of functioning mitochondria. |
|
Response of ZF4 cells to hypoxic stress. |