- Title
-
PPP6C, a serine-threonine phosphatase, regulates melanocyte differentiation and contributes to melanoma tumorigenesis through modulation of MITF activity
- Authors
- Maskin, C.R., Raman, R., Houvras, Y.
- Source
- Full text @ Sci. Rep.
|
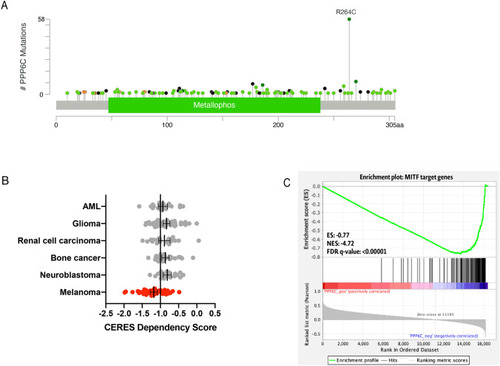
PPP6C is recurrently mutated in melanoma and has a unique relationship to melanocyte lineage and MITF. ( |
|
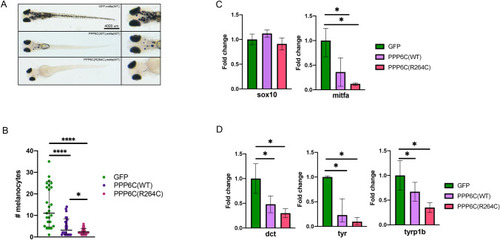
PPP6C disrupts melanocyte differentiation in vivo |
|
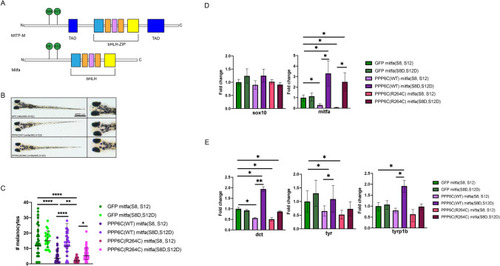
MITF mutation prevents PPP6C-mediated melanocyte differentiation disruption |
|
MITF promoter activity is reduced under expression of PPP6C(WT) and PPP6C(R264C) on multiple oncogenic backgrounds. ( |
|
The recurrent |
|
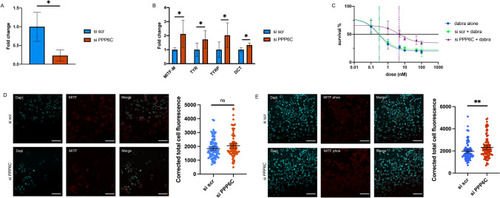
PPP6C expression affects MITF expression and drug resistance in melanoma. ( |