- Title
-
Glucose inhibits haemostasis and accelerates diet-induced hyperlipidaemia in zebrafish larvae
- Authors
- Morris, S., Cholan, P.M., Britton, W.J., Oehlers, S.H.
- Source
- Full text @ Sci. Rep.
|
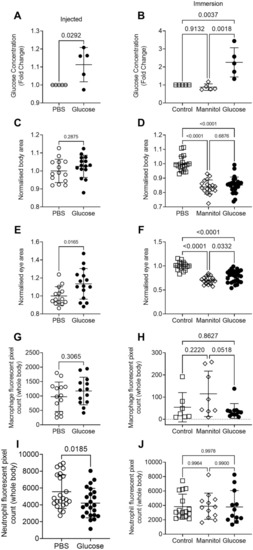
Injection and immersion methods increase glucose levels in zebrafish larvae. ( |
|
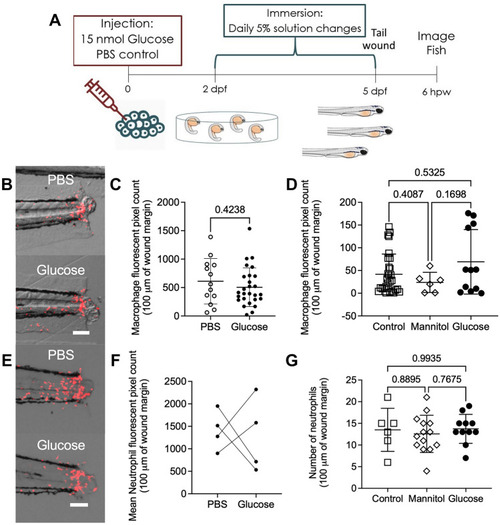
Exogenous glucose does not affect neutrophil and macrophage recruitment to a tail wound. ( |
|
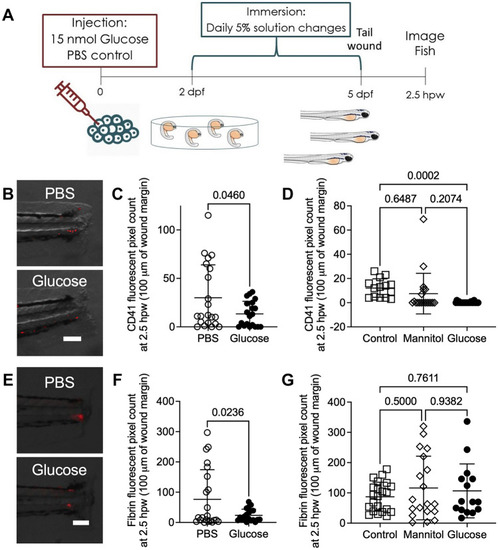
Exogenous glucose supplementation reduced thrombocyte and fibrin accumulation at a tail wound. ( |
|
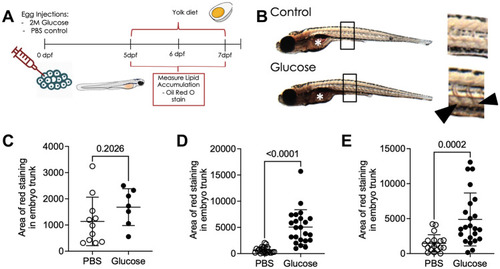
Glucose-injected larvae have increased lipid accumulation following a high fat diet. ( |