- Title
-
Potential of peptide-engineered exosomes with overexpressed miR-92b-3p in anti-angiogenic therapy of ovarian cancer
- Authors
- Wang, J., Wang, C., Li, Y., Li, M., Zhu, T., Shen, Z., Wang, H., Lv, W., Wang, X., Cheng, X., Xie, X.
- Source
- Full text @ Clin Transl Med
|
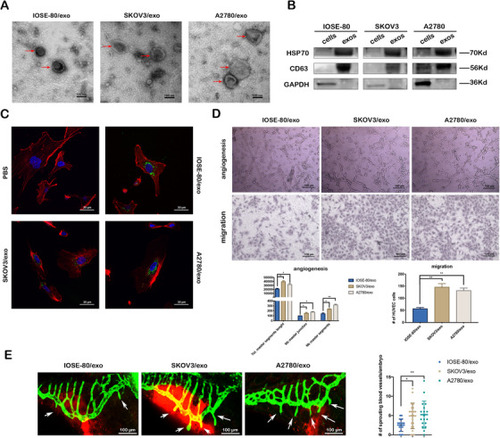
Cancer cell‐derived exosomes promote the angiogenesis and migration viability of HUVECs in vitro and in vivo. (A) Representative TEM images of exosomes (red arrows) (scale bar = 100 nm). (B) Western blotting of the whole‐cell lysates or exosome lysates for the classical exosomal protein markers (CD63 and Hsp70) and GAPDH. (C) Representative confocal microscope images of F‐actin (red), nucleus (blue), and PKH67‐labeled exosomes (green) in HUVECs co‐cultured with various exosomes or PBS for 24 h (scale bar = 30 μm). (D) Representative images of tube formation and migration of HUVECs treated by IOSE‐80/exo, SKOV3/exo, and A2780/exo, respectively (scale bar = 100 μm). Total master segments length, number of master junctions, master nodes, and migration cells were regarded as indicators of angiogenic ability in vitro and assessed by ImageJ (mean ± SD, |
|
miR‐92b‐3p acts as a suppressor of tumor‐associated angiogenesis. (A) Venn diagram of the overlapping differentially expressed miRNAs ( |
|
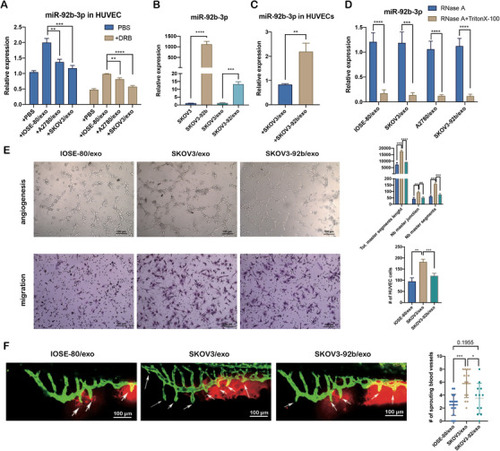
Exosomal miR‐92b‐3p modulates tumor‐associated angiogenesis. (A) qRT‐PCR analysis on the relative expression of miR‐92b‐3p in HUVECs treated with IOSE‐80/exo, SKOV3/exo, and A2780/exo, respectively, for 48 h with or without 5,6‐dichlorobenzimidazole 1‐β‐D‐ribofuranoside (DRB) (20 μm/ml), compared to HUVECs treated with PBS (mean ± SD, |
|
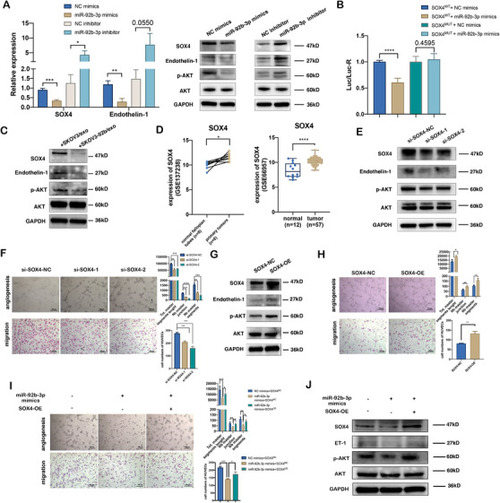
SOX4 is a target of exosomal miR‐92b‐3p and participates in exosomal miR‐92b‐3p modulating angiogenesis. (A) qRT‐PCR analysis (left) and Western blotting (right) of the relative expressions of SOX4, endothelin‐1, and p‐AKT in HUVECs transfected with miR‐92b‐3p mimics or miR‐92b‐3p inhibitor (mean ± SD, |
|
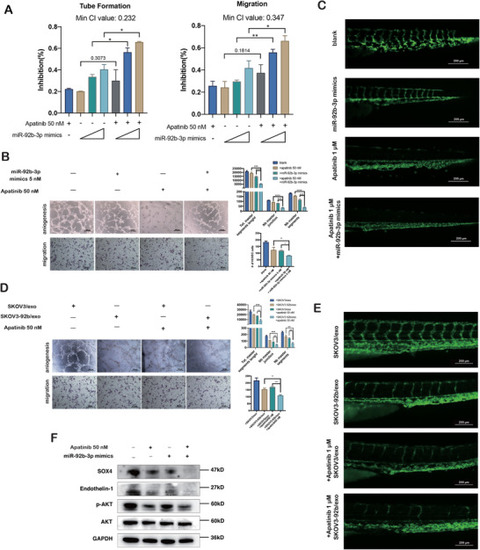
Exosomal miR‐92b‐3p and Apatinib represent the synergistic anti‐angiogenic effect. (A) Inhibition rates of tube formation and migration of HUVECs treated with Apatinib or miR‐92b‐3p mimics alone or both combined (mean ± SD, |
|
Engineered RGD‐SKOV3‐92b/exo alone or combined with Apatinib inhibits tumor growth in vivo. (A) Representative confocal microscopy images of HUVECs treated with SKOV‐92b/exo or RGD‐SKOV3‐92b/exo. F‐actin (red), nucleus (blue), and PKH67‐labeled exosomes (green) were stained (scale bar = 30 μm). Relative uptake was calculated using ImageJ software. (B) Total fluorescence values of nude mice in different groups. There were no differences among randomization groups for any of the characteristics. (C) Schematic protocol of intraperitoneal xenografted tumors in animal experiments. (D) Bioluminescence images of nude mice with different exosomal injections or Apatinib treatment. Total fluorescence values were assessed by fluorescence imaging ( |
|
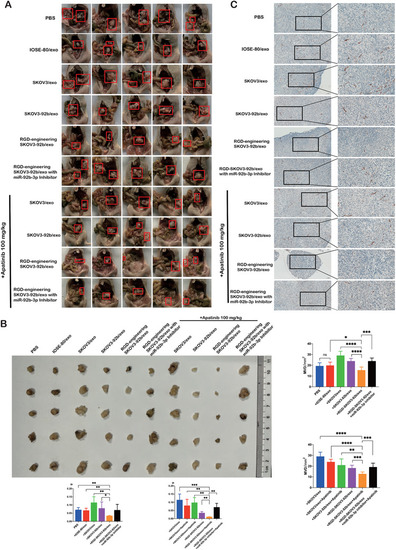
Tumor volume and angiogenesis were inhibited by engineered RGD‐SKOV3‐92b/exo alone or combined with Apatinib. (A) Gross anatomy images of nude mice in the peritoneal cavity. (B) Schematic representation of intraperitoneal xenografted tumors in different groups. Tumor size from each group was measured and weighed as represented. (C) Immunohistochemical staining of CD31 in intraperitoneal xenografted tumors (scale bar = 100 μm and 50 μm). Micro‐vessels density (MVD) was assessed by counting CD31‐positive cells ( |