- Title
-
A novel role of Zebrafish TMEM33 in negative regulation of interferon production by two distinct mechanisms
- Authors
- Lu, L.F., Zhang, C., Li, Z.C., Zhou, X.Y., Jiang, J.Y., Chen, D.D., Zhang, Y.A., Xiong, F., Zhou, F., Li, S.
- Source
- Full text @ PLoS Pathog.
|
(A-F) qPCR detection of the transcriptional levels of |
|
(A and B) Overexpression of TMEM33 suppresses poly I:C/SVCV-induced IFNφ1pro/ISRE-Luc activation and displays a dose-dependent manner. EPC cells were seeded in 24-well plates and transfected the next day with 250 ng IFNφ1pro-Luc (A) or ISRE-Luc (B) and 25 ng pRL-TK, plus pcDNA3.1-TMEM33 (250 ng or 200/400 ng) or pcDNA3.1(+) (control vector). At 24 h post-transfection, cells were untreated (null) or transfected with poly I:C (1 μg/ml) or treated with SVCV (MOI = 1). Luciferase activities were monitored at 24 h after stimulation. The promoter activity is presented as relative light units (RLU) normalized to |
|
(A and B) Enhance of virus replication by overexpression of TMEM33. EPC cells seeded in 24-well plates overnight were transfected with 0.5 μg of TMEM33-Myc or empty vector. At 24 h post-transfection, cells were infected with SVCV (MOI = 0.001) for 48 h. Then, cells were fixed with 4% PFA and stained with 1% crystal violet (A). Culture supernatants from the cells infected with SVCV were collected, and the viral titer was measured according to the method of Reed and Muench (B). (C-G) Overexpression of TMEM33 inhibits the expression of |
|
(A and B) EPC cells were seeded into 24-well plates overnight and co-transfected with MAVS-, MITA-, or TBK1-expressing plasmid and empty vector or TMEM33-Myc (250 ng or 250/500 ng), plus IFN?1pro-Luc (A and C) or ISRE-Luc (B and D) at the ratio of 1:1:1. pRL-TK was used as a control. At 24 h post-transfection, cells were lysed for luciferase activity detection. Data were expressed as mean ± SEM, n = 3. Asterisks indicate significant differences from control (*, p < 0.05). |
|
(A) TMEM33 interacts with MAVS, MITA, TBK1, and IRF3. EPC cells seeded in 10 cm2 dishes were transfected with the indicated plasmids (5 ?g each). After 24 h, cell lysates were immunoprecipitated (IP) with anti-Flag affinity gel. Then the immunoprecipitates and WCLs were analyzed by IB with the anti-Myc and anti-Flag Abs, respectively. (B and C) TMEM33 is localized at the ER. EPC cells seeded onto microscopy cover glass in 6-well plates were co-transfected with 1 ?g TMEM33-EGFP and 1 ?g empty vector (B) or ER-DsRed (C). After 24 h, the cells were fixed and subjected to confocal microscopy analysis. Green signals represent overexpressed TMEM33, and blue staining indicates the nucleus region. The yellow staining in the merged image indicates that TMEM33 is localized at the ER. (D-G) TMEM33 colocalizes with MAVS and MITA. EPC cells were plated onto coverslips in 6-well plates and co-transfected with 1 ?g TMEM33-EGFP and 1 ?g MAVS-DsRed (D), TBK1-DsRed (E), MITA-DsRed (F), or IRF3-DsRed (G). After 24 h, the cells were fixed and observed by confocal microscopy. Green signals represent overexpressed TMEM33. Red signals represent overexpressed MAVS, TBK1, MITA, or IRF3, and blue staining indicates the nucleus region. The yellow staining in the merged image indicates the colocalization of TMEM33 and MAVS or MITA (original magnification 63×; oil immersion objective). Scale bar, 10 ?m. (H) The colocalization rates of B-G were performed by LAS AF Lite. All experiments were repeated for at least three times with similar results. |
|
(A) Schematic representation of full-length TMEM33 and its mutants. (B-D) The first and the second TM regions of TMEM33 are necessary for its association with MAVS, TBK1, and MITA. HEK 293T cells seeded in 10 cm2 dishes were co-transfected with the indicated plasmids (5 ?g each). After 24 h, cell lysates were immunoprecipitated (IP) with anti-Flag affinity gel (B and C) or anti-Myc affinity gel (D). Then the immunoprecipitates and WCLs were analyzed by IB with the indicated Abs. (E-G) Diagrammatic representations of full-length MAVS/TBK1/MITA and deletion mutants in this study. (H) The N-terminal CARD domain and C-terminal TM domain of MAVS are responsible to its interaction with TMEM33. The experiments were performed similarly as described above for panel B. (I) The N-terminal kinase domain of TBK1 is essential to its interaction with TMEM33. The experiments were performed similarly as described above for panel B. (J) TMEM33 associates with MITA via its N terminus. The experiments were performed similarly as described above for panel B. All experiments were repeated for at least three times with similar results. |
|
(A-C) Overexpression of TMEM33 degrades MAVS in a dose-dependent manner. EPC cells were seeded in 6-well plates overnight and co-transfected with 1 ?g TMEM33-HA and 1 ?g empty vector, MAVS-Myc, MITA-Myc, or TBK1-Myc (A); TMEM33-HA (1 or 2 ?g) and 1 ?g MAVS-Myc (B) or MITA-Myc (C) for 24 h. The cell lysates were subjected to IB with anti-Myc, anti-HA, and anti-?-actin Abs. (D and E) TMEM33-?TM1 and TMEM33-?TM2 have no effect on the exogenous MAVS and MITA. EPC cells were seeded in 6-well plates overnight and transfected with the indicated plasmids (1 ?g each) for 24 h. The cell lysates were subjected to IB with the indicated Abs. (F) TMEM33 has no influences on the poly I:C/SVCV-induced transcriptions of MAVS. EPC cells were transfected with 2 ?g TMEM33-Myc or empty vector for 24 h, and then transfected with poly I:C or infected with SVCV (MOI = 1) for 24 h. Total RNAs were extracted to examine the mRNA level of mavs by qPCR. The relative transcriptional levels were normalized to the transcription of ?-actin and represented as fold induction relative to the transcriptional level in the control cells, which was set to 1. Data were expressed as mean ± SEM, n = 3. (G) Effects of inhibitors on TMEM33-mediated destabilization of MAVS. EPC cells were seeded in 6-well plates overnight and co-transfected the indicted plasmids. At 18 h post-transfection, the cells were treated with DMSO, MG132 (20 ?M), 3-MA (2 mM), or NH4Cl (20 mM) for 6 h. The cell lysates were subjected to IB with the indicated Abs. (H) TMEM33-induced MAVS degradation is rescued by MG132 in a dose-dependent manner. EPC cells were seeded in 6-well plates overnight and co-transfected the indicted plasmids. At 18 h post-transfection, the cells were treated with DMSO or MG132 (10, 20, or 40 ?M) for 6 h. Then, the cells were harvested for IB with the indicated Abs. (I) TMEM33 promotes the ubiquitination of MAVS. EPC cells were transfected with 5 ?g MAVS-Myc, 5 ?g TMEM33-HA or empty vector, and 1 ?g Ub-HA. At 18 h post-transfection, the cells were treated with DMSO or MG132 for 6 h. Cell lysates were IP with anti-Myc affinity gel. Then the immunoprecipitates and WCLs were analyzed by IB with the indicated Abs. (J) TMEM33 mediates K48-linked ubiquitination of MAVS in vivo. EPC cells were transfected with 5 ?g MAVS-Myc, 5 ?g TMEM33-HA or empty vector, and 1 ?g Ub-HA, Ub-K48O-HA or Ub-K63O-HA. At 18 h post-transfection, the cells were treated with MG132 for 6 h. At 24 h post-transfection, cell lysates were IP with anti-Myc-affinity gel. Then the immunoprecipitates and WCLs were analyzed by IB with the indicated Abs. All experiments were repeated for at least three times with similar results. |
|
(A and B) Overexpression of TMEM33 restores MAVS-mediated decrease of viral titer. EPC cells seeded in 24-well plates overnight were transfected with 0.25 ?g of MAVS-Myc and 0.25 ?g of TMEM33-HA or empty vector. At 24 h post-transfection, cells were infected with SVCV (MOI = 0.001) for 48 h. Then, cells were fixed with 4% PFA and stained with 1% crystal violet (A). Culture supernatants from the cells infected with SVCV were collected, and the viral titer was measured according to the method of Reed and Muench (B). (C) EPC cells were seeded in 6-well plates overnight and then transfected with 2 ?g of MAVS-Myc and 2 ?g of TMEM33-HA or empty vector. At 24 h post-transfection, cells were infected with SVCV (MOI = 1). After 24 h-infection, total RNAs were extracted to examine the mRNA levels of cellular g, l, m, n, and p. The relative transcriptional levels were normalized to the transcriptional level of the ?-actin gene and were represented as fold induction relative to the transcriptional level in the control cells, which was set to 1. Data were expressed as mean ± SEM, n = 3. Asterisks indicate significant differences from control values (*, p < 0.05). (D) The same samples were prepared similarly as described above for panel C. The lysates were detected by IB with the anti-N, anti-P, anti-Myc, anti-HA, and anti-?-actin Abs, respectively. (E) TMEM33 enhances the SVCV-induced K48-linked ubiquitination of MAVS. EPC cells were transfected with 5 ?g MAVS-Myc, 5 ?g TMEM33-HA or empty vector, and 1 ?g Ub-HA, Ub-K48O-HA or Ub-K63O-HA. At 24 h post-transfection, the cells were uninfected or infected with SVCV (MOI = 1) for 18 h, then the cells were treated with MG132 for 6 h. Cell lysates were IP with anti-Myc-affinity gel. Then the immunoprecipitates and WCLs were analyzed by IB with the indicated Abs. (F-J) Overexpression of TMEM33 inhibits the expression of ifn (F), vig1 (G), isg15-1 (H), irf7 (I), and rig-i (J) induced by MAVS. EPC cells seeded in 6-well plates overnight were transfected with 2 ?g of TMEM33-HA or empty vector together with 2 ?g of MAVS-Myc or empty vector. At 24 h after transfection, total RNAs were extracted to examine the mRNA levels of cellular ifn, vig1, isg15-1, irf7, and rig-i. The relative transcriptional levels were normalized to the transcriptional level of the ?-actin gene and were represented as fold induction relative to the transcriptional level in the control cells, which was set to 1. (K and L) TMEM33-?TM1 and TMEM33-?TM2 have no effect on MAVS-mediated suppression of viral genes transcription and protein expression. EPC cells were seeded in 6-well plates overnight and transfected with the indicated plasmids (2 ?g each) for 24 h. At 24 h post-transfection, cells were infected with SVCV (MOI = 1) for 24 h. qPCR and immunoblot analysis were performed similarly as in C and D. |
|
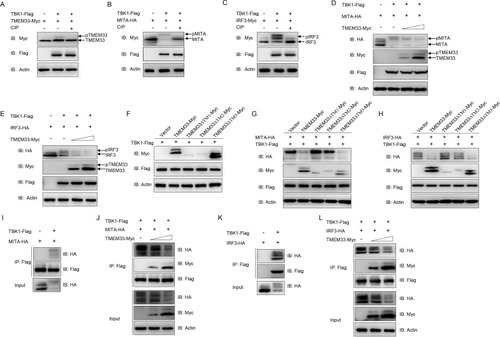
(A) The amount of TBK1-phosphorylated TMEM33 is reduced by CIP treatment. EPC cells were seeded in 6-well plates overnight and transfected with the indicated plasmids (1 μg each) for 24 h. The cell lysates (100 μg) were treated with or without CIP (10 U) for 40 min at 37°C. Then the lysates were detected by IB with the indicated Abs. (B and C) TBK1 mediates the phosphorylation of MITA (B) and IRF3 (C). EPC cells were seeded into 6-well plates overnight and transfected with the indicated plasmids (1 μg each) for 24 h. The cell lysates (100 μg) were treated with or without CIP (10 U) for 40 min at 37°C. The lysates were then subjected to IB with the indicated Abs. (D and E) Overexpression of TMEM33 inhibits TBK1-mediated phosphorylation of MITA (D) and IRF3 (E) in a dose-dependent manner. EPC cells were seeded into 6-well plates overnight and co-transfected with 1 μg TBK1-Flag and 1 μg empty vector or TMEM33-Myc (0.5 and 1 μg, respectively), together with 1 μg MITA-HA or IRF3-HA for 24 h. The lysates were then subjected to IB with the indicated Abs. (F) TBK1 phosphorylates wild-type TMEM33 and TMEM33-ΔTM3. EPC cells were seeded in 6-well plates overnight and transfected with the indicated plasmids (1 μg each) for 24 h. Then the lysates were detected by IB with the indicated Abs. (G and H) TMEM33-ΔTM1 and TMEM33-ΔTM2 have no effect on TBK1-mediated phosphorylation of MITA (G) and IRF3 (H). EPC cells were seeded into 6-well plates overnight and co-transfected with 1 μg TBK1-Flag and 1 μg empty vector, TMEM33-Myc, TMEM33-ΔTM1-Myc, TMEM33-ΔTM2-Myc, or TMEM33-ΔTM3-Myc together with 1 μg MITA-HA or IRF3-HA for 24 h. The lysates were then subjected to IB with the indicated Abs. (I and J) TMEM33 blocks the interaction between TBK1 and MITA in a dose-dependent manner. EPC cells seeded in 10 cm2 dishes were co-transfected with 4 μg TBK1-Flag and 4 μg MITA-HA (I) or together with TMEM33-Myc (2 or 4 μg) (J). After 24 h, cell lysates were immunoprecipitated (IP) with anti-Flag affinity gel. Then the immunoprecipitates and WCLs were analyzed by IB with the indicated Abs. (J and K) TMEM33 disrupts the interaction between TBK1 and IRF3 in a dose-dependent manner. EPC cells seeded in 10 cm2 dishes were co-transfected with 4 μg TBK1-Flag and 4 μg IRF3-Myc (J) or together with TMEM33-HA (2 or 4 μg) (K) for 24 h. Immunoprecipitation and immunoblot analysis were performed similarly as in I and J. All experiments were repeated for at least three times with similar results. |
|
(A and B) Overexpression of TMEM33 increases TBK1-mediated decline of viral titer. EPC cells seeded in 24-well plates overnight were transfected with 0.25 μg of TBK1-Myc and 0.25 μg of TMEM33-HA or empty vector. At 24 h post-transfection, cells were infected with SVCV (MOI = 0.001) for 48 h. Then, cells were fixed with 4% PFA and stained with 1% crystal violet (A). Culture supernatants from the cells infected with SVCV were collected, and the viral titer was measured according to the method of Reed and Muench (B). (C) EPC cells seeded in 6-well plates overnight were transfected with 2 μg of TMEM33-HA or empty vector together with 2 μg of TBK1-Myc or empty vector. At 24 h post-transfection, cells were infected with SVCV (MOI = 1). After 24 h-infection, total RNAs were extracted to examine the mRNA levels of cellular |
|
(A and B) TMEM33-ΔTM1 and TMEM33-ΔTM2 have no effect on poly I:C/SVCV-induced IFNφ1pro/ISRE-Luc activation. EPC cells were seeded in 24-well plates and transfected the next day with 250 ng IFNφ1pro-Luc (A) or ISRE-Luc (B) and 25 ng pRL-TK, plus 250 ng TMEM33-Myc, TMEM33-ΔTM1-Myc, TMEM33-ΔTM2-Myc, TMEM33-ΔTM3-Myc, or pCMV-Myc (control vector). At 24 h post-transfection, cells were untreated (null) or transfected with poly I:C (1 μg/ml) or treated with SVCV (MOI = 1). Luciferase activities were monitored at 24 h after stimulation. The promoter activity is presented as relative light units (RLU) normalized to |
|
Upon virus infection, fish RIG-I senses the viral RNA and interacts with MAVS, leading to the recruitment of TBK1, which phosphorylates MITA and IRF3, then induces IFN production. TMEM33, an ER-localized protein, triggers the K48-linked ubiquitination and degradation of MAVS, meanwhile, it also reduces MITA/IRF3 phosphorylation by acting as a substrate of TBK1, thereby preventing IFN expression. |