- Title
-
Monitoring Tuberculosis Drug Activity in Live Animals by Near-Infrared Fluorescence Imaging
- Authors
- Sommer, R., Cole, S.T.
- Source
- Full text @ Antimicrob. Agents Chemother.
|
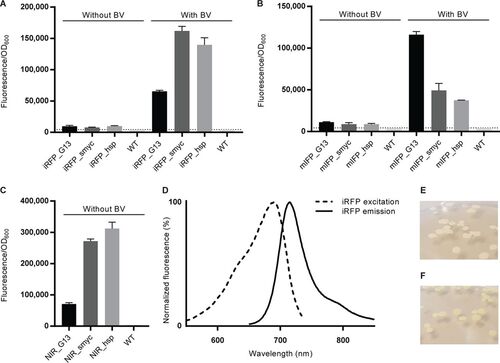
M. smegmatis reporter strains emit NIR fluorescence. (A to C) Fluorescence intensity measured at 713 nm for M. smegmatis expressing iRFP (A) or mIFP (B) and cultured overnight with or without BV or for NIR fluorescent M. smegmatis reporters without BV supplementation (C). The fluorescence intensity was measured with a microplate reader and normalized according to the cell density for each well. The dotted lines indicate the background autofluorescence recorded in WT bacteria under the same conditions. Data represent the averages ± SEM for 4 independent clones per group. (D) Fluorescence excitation and emission spectra of the M. smegmatis NIR_smyc reporter. (E, F) Photographs of colonies of WT M. smegmatis (E) and M. smegmatis NIR_smyc reporter bacteria (F), with the latter displaying a faint green color. |
|
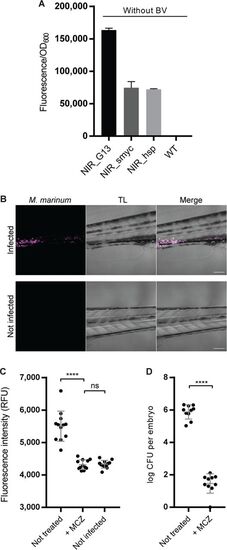
NIR fluorescent M. marinum can be visualized and quantified in infected zebrafish embryos. (A) Measurement of NIR fluorescence in cultures of M. marinum transformed with the indicated reporter constructs; data are for 3 independent transformants per measurement. The bars show the averages ± SEM. (B) Single-plane confocal micrographs showing NIR fluorescent M. marinum in zebrafish embryos at 3 dpi. Bacteria are visualized in NIR fluorescence (left), a gray picture (middle) is taken with transillumination (TL), and merged channels are shown at the right. The bottom panels are for an uninfected larva, highlighting the specific fluorescence emitted by the bacteria (displayed in magenta). Bars, 100 ?m. (C) NIR fluorescence measurement of live infected zebrafish embryos with or without MCZ treatment. RFU, relative fluorescence units. (D) The numbers of CFU recovered from infected embryos with or without MCZ treatment. Each dot corresponds to one embryo, and data are for 12 (C) or 10 (D) elements per group. Bars show averages and standard deviations. ****, P < 0.0001; ns, not significant. |
|
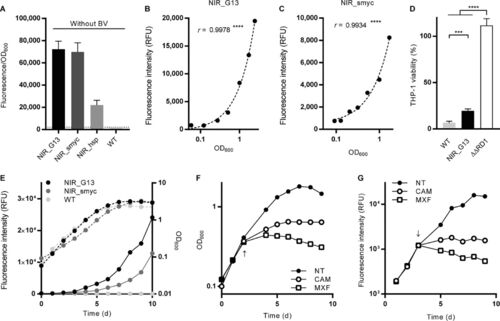
Characterization of NIR fluorescent reporters in M. tuberculosis. (A) Higher fluorescence intensities can be achieved when iRFP and ho1 are expressed from the G13 or Psmyc promoter. The dotted line indicates the background fluorescence of WT bacteria under the same measurement conditions. (B, C) The NIR fluorescence measured over 1 week of growth in liquid culture correlates with the OD600 for the M. tuberculosis NIR_G13 (B) and NIR_smyc (C) strains. r is the Pearson correlation coefficient. ****, P < 0.0001. (D) Viability of THP-1 cells upon infection with M. tuberculosis NIR_G13, the parental (WT) strain, and the attenuated ??RD1 strain. ***, P < 0.001; ****, P < 0.0001. (E) Comparison of the growth curves (broken line) and evolution of the fluorescence intensity (solid lines) in liquid cultures for the NIR_G13, NIR_smyc, and parental (WT) strains. (F) Measurements of the cell density in M. tuberculosis NIR_G13 liquid cultures exposed to chloramphenicol (CAM), moxifloxacin (MXF), or no treatment (NT). (G) Measurements of NIR fluorescence in M. tuberculosis NIR_G13 liquid cultures exposed to the indicated antibiotics or no treatment. The time of addition of antibiotics is indicated by an arrow. d, day. |
|
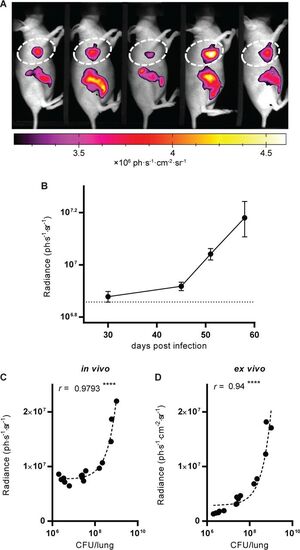
Visualization and quantification of NIR fluorescence in living infected animals. (A) One group of mice analyzed at 45 dpi with NIR fluorescence. The picture is a black-and-white photograph, and the NIR fluorescent signal is overlaid in false color. The quantified zone in the thorax is delimited by dashed lines, and the results obtained at 45 dpi are reported in panel B. (B) Quantification of the fluorescence intensity in the thorax of different groups of mice at the time points indicated. Data show the averages ± SEM. The dotted line indicates the average background fluorescence level measured in uninfected mice. For all time points, data are for 5 mice per group. (C, D) The number of CFU recovered from the lungs of mice at 30, 45, and 58 dpi correlates with the fluorescence measured and quantified in vivo (C) and ex vivo on excised lungs (D). r is the Pearson correlation coefficient. ****, P < 0.0001. The dashed lines are linear regressions. |
|
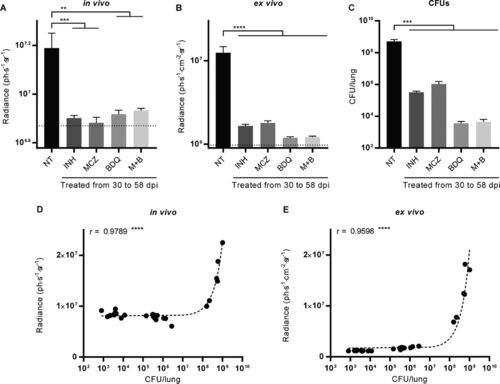
Fluorescence measurements and CFU enumeration to assess antibiotic efficacy. (A, B) Fluorescence was measured in vivo and quantified in the thorax of mice (A) or postmortem on excised lungs (B). For the latter, the fluorescence intensity measurement is adjusted for the area of the lung on which the quantification was done. (C) Serial 10-fold dilutions of lung homogenates were plated, and the CFU were enumerated after 4 to 5 weeks of growth at 37°C. The dotted line indicates the average background fluorescence measured in uninfected mice (A) and lungs (B). Bars indicate the average ± SEM. Data are for 5 mice in each group. NT, no treatment; INH, isoniazid; MCZ, macozinone; BDQ, bedaquiline; M+B, a combination of macozinone and bedaquiline. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. (D, E) The number of CFU recovered at the end of the experiment from the lungs of untreated and treated mice correlates with the fluorescence measured and quantified in vivo (D) and ex vivo on excised lungs (E). Each point corresponds to a measurement for one mouse, r is the Pearson correlation coefficient, and dashed lines are linear regressions. ****, P < 0.0001. |