- Title
-
IL-10 from plasmacytoid dendritic cells promotes angiogenesis in the early stage of endometriosis
- Authors
- Suen, J.L., Chang, Y., Shiu, Y.S., Hsu, C.Y., Sharma, P., Chiu, C.C., Chen, Y.J., Hour, T.C., Tsai, E.M.
- Source
- Full text @ J. Pathol.
|
The effect of IL‐10‐expressing pDCs on endometriosis development in a surgically induced model. (A) Lesion area and weight from C57BL/6 mice 4 weeks after surgery. The mice were locally treated at the implantation site as indicated. rmIL‐10, recombinant murine IL‐10; IC, isotype control; αmIL‐10, mAb against mouse IL‐10. |
|
Quantification of CD31+ cells in endometriotic lesions in the murine model. (A) Representative immunofluorescence sections of lesions from PBS‐ or rmIL‐10‐treated mice. Blue, nucleus (DAPI); red, CD31 (Alexa Fluor 568). Red scale bars = 20 µm. (B) Representative dot plots analysed by TissueQuest software. (C) The frequency of CD31+ cells among DAPI+ cells in the whole field of each lesion. IC, isotype control; αmIL‐10, mAb against mouse IL‐10. One dot represents one section from each endometriotic lesion. One endometriotic lesion was sampled per mouse. |
|
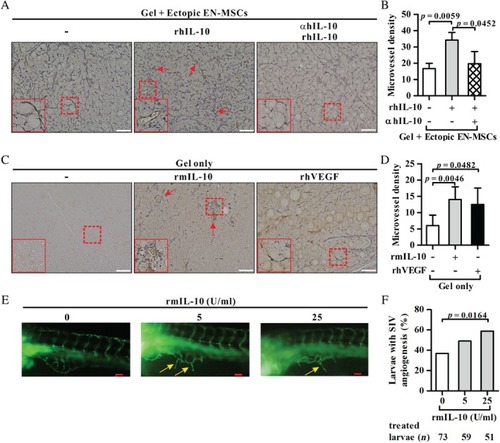
Effect of the IL‐10—IL‐10R pathway on the angiogenic activity of HUVECs |
|
Effect of the IL‐10—IL‐10R pathway on angiogenesis |
|
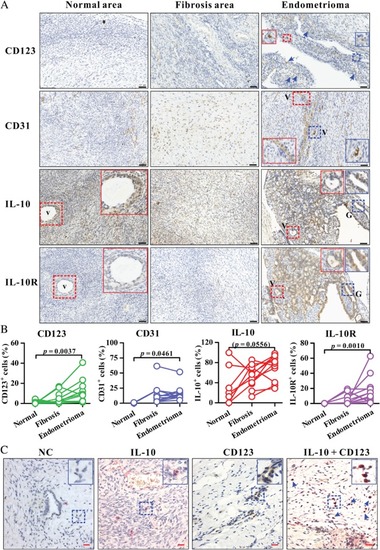
Analysis of CD123, CD31, IL‐10, and IL‐10R expression in human endometrioma and uterine endometrial tissues. (A) Immunohistochemistry for CD123, CD31, IL‐10, or IL‐10R (brown colour) in a representative endometrioma lesion as well as the fibrosis area and normal area surrounding the corresponding endometrioma lesion. The insets (solid box) show two‐fold enlarged images of vascular structure (V) or glandular epithelium (G) (dashed box) with the same colour. Arrows indicate CD123+ cells. Blue, haematoxylin counterstain. Black scale bars = 50 µm. The enlarged images of IL‐10 and IL‐10R expression on endometrioma lesions in A are shown in supplementary material, Figure |
|
Schematic representation of the effect and immune cell sources of IL‐10 during the early and late stages of endometriosis. During the early implantation and invasion stages, lesion pDCs may secrete IL‐10 in response to unwanted and apoptotic cells. Local IL‐10 further promotes lesion growth by either suppressing anti‐ectopic fragment immunity or stimulating angiogenesis in VEGF‐dependent and ‐independent pathways. In addition, IL‐10—IL‐10R signalling may stimulate endometrial cell migration. The complex interaction among genetic factors, endogenous hormones, environmental endocrine disruptors (EEDs), and impaired immune surveillance leads to chronic inflammation. In the context of chronic inflammation in the peritoneal cavity, other immune cells, such as alternative activated macrophages and Th17 cells as well as endometrial cells (not shown here), can secrete IL‐10 and other mediators to further promote the growth and maintenance of ectopic implants. |