- Title
-
Dietary cholesterol and apolipoprotein A-I are trafficked in endosomes and lysosomes in the live zebrafish intestine
- Authors
- Otis, J.P., Shen, M.C., Caldwell, B.A., Reyes Gaido, O.E., Farber, S.A.
- Source
- Full text @ Am. J. Physiol. Gastrointest. Liver Physiol.
|
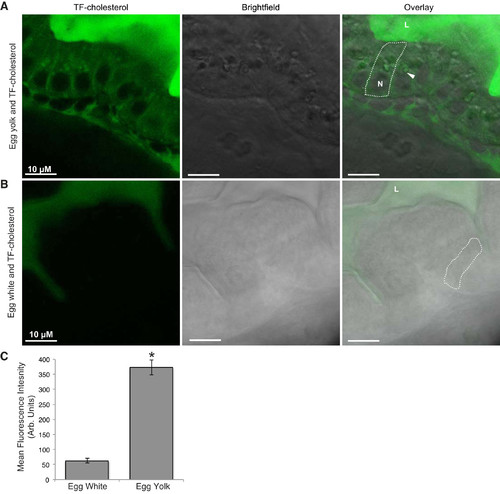
Visualization of TF-cholesterol absorption in live, larval zebrafish indicates fatty acids are necessary for dietary cholesterol absorption. TF-cholesterol provided in a lipid-rich meal (5% chicken egg yolk) is absorbed by enterocytes and accumulates in distinct subcellular punctae (A). Representative images show that dietary TF-cholesterol absorption is not observed when it is fed with a lipid-poor meal (5% chicken egg white) (B). Approximately sixfold more TF-cholesterol fluorescence is observed in enterocytes when it is provided with egg yolk relative to egg white (C) (means ± SE, student’s t-test, *P < 0.05). n = 3, with 6–9 fish per n. Arrows indicate colocalization; L, intestinal lumen; N, nucleus; TF-cholesterol, TopFluor-cholesterol.
|
|
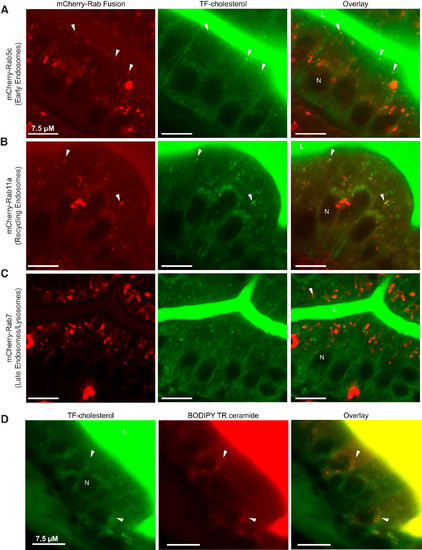
Dietary TF-cholesterol localizes to the endosomal-lysosomal trafficking network in enterocytes. Representative images show that 3 h following the onset of feeding, dietary TF-cholesterol colocalizes to mCherry-Rab5c (early endosomes) (A), mCherry-Rab11a (recycling endosomes) (B), and mCherry-Rab7 (late endosomes) (C). n = 3, with 3–9 fish per n; all fish 6-dpf. TF-cholesterol colocalizes with BODIPY TR ceramide, which marks the trans-Golgi network, in enterocytes after 4 h of feeding (D). n = 3, with 3–6 fish per n. Arrows indicate colocalization; dpf, days postfertilization; L, intestinal lumen; N, nucleus; TF-cholesterol, TopFluor-cholesterol.
|
|
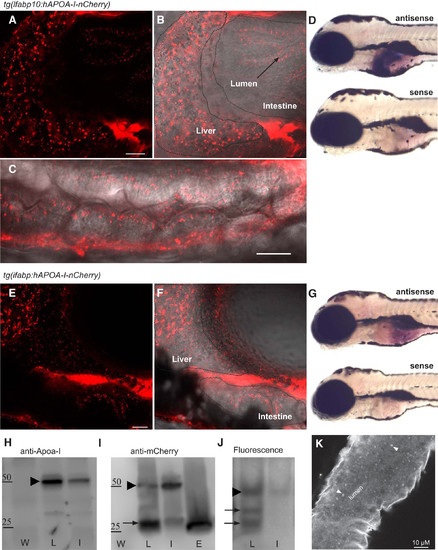
Transgenic zebrafish allows for visualization of human APOA-I in vivo. Live tg(lfabp10:hAPOA-I-mCherry) (A–C) and tg(ifabp:hAPOA-I-mCherry) (E–F) larvae show hAPOA-I-mCherry accumulation in the liver and intestine. hAPOA-I-mCherry derived from the liver (outlined by dotted line) accumulates in the intestine outlined by wavy line) and hAPOA-I-mCherry derived from the intestine accumulates in the liver (B and C). Tissue specificity of promoters used in APOA-I-mCherry transgenic fish revealed using whole mount in situ hybridization with antisense riboprobes to mCherry mRNA (D and G). No expression was detected with the sense probes (n = 10–12 fish); all larvae 6-dpf. ApoA-I-mCherry fusion protein is made in transgenic zebrafish and partially degraded (H). ApoA-I-mCherry fusion protein (arrow head) is made in transgenic zebrafish as expected size (50 kDa) (representative image from three experiments). mCherry antisera detected two different bands from Tg(lfabp:hApoA-I-mcherry) and Tg(ifabp:hApoA-I-mcherry) (I). The higher molecular weight bands (arrow head) represent ApoA-I-mCherry full-length protein. The lower molecular weight bands (arrow) is possibly a degradation product of similar size as mCherry protein made from Tg(ef1a:mcherry-CVLL) (representative image from two experiments). mCherry flourscence on native gel (representative image from four experiments) (J). Immunofluorescence for endogenous zApoA-Ia and zApoA-Ib in wild-type fish show punctae within the intestine similar to hAPOA-I-mCherry accumulation (representative image from three experiments) (K). APOA-I, apolipoprotein A-I; dpf, days postfertilization; E, Tg(ef1a:mcherry-CVLL); hAPOA-I, human APOA-I; I, Tg(ifabp:hApoA-I-mcherry); L, Tg(lfabp:hApoA-I-mcherry); W, wild-type; zAPOA-1, zebrafish APOA-1. |
|
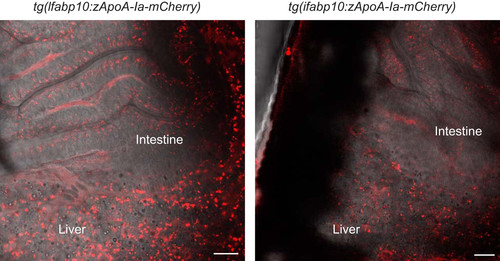
Transgenic zebrafish allows for visualization of zebrafish APOA-Ia in vivo. Live tg(lfabp10:zApoA-Ia-mCherry) (left) and tg(ifabp:zApoA-Ia-mCherry) larvae show APOA-I-mCherry accumulation in the liver and intestine (right). Scale bar = 20 µm, all larvae 6-dpf. APOA-I, apolipoprotein A-I; dpf, days postfertilization. |
|
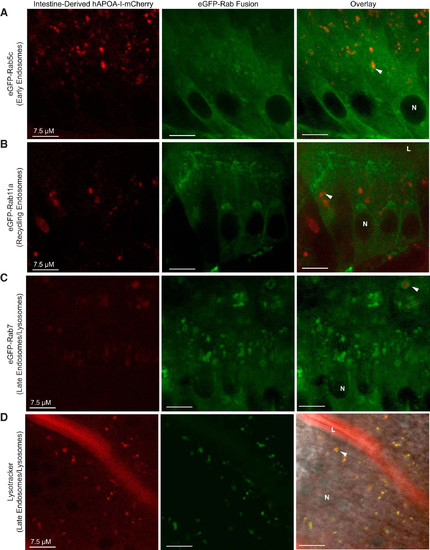
hAPOA-I-mCherry localizes to the endosomal-lysosomal trafficking system in enterocytes of tg(ifabp:hAPOA-I-mCherry) larvae. Representative images demonstrate that hAPOA-I-mCherry colocalizes to early endosomes (A), recycling endosomes (B), and late endosomes/lysosomes, as marked by eGFP-Rab7 (C) and Lysotracker (D), in enterocytes. Arrowheads: colocalization; n = 3–4, with 3–12 fish per n, all fish 6-dpf. APOA-I, apolipoprotein A-I; dpf, days postfertilization; hAPOA-I, human APOA-I; L, intestinal lumen; N, nucleus. |
|
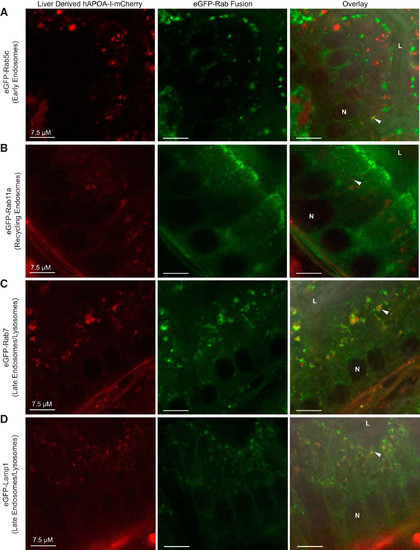
hAPOA-I-mCherry localizes to the endosomal-lysosomal trafficking system in enterocytes of tg(lfabp10:hAPOA-I-mCherry) larvae. Representative images showing hAPOA-I-mCherry colocalized to early endosomes (eGFP-Rab5c) (A), recycling endosomes (eGFP-Rab11a) (B), late endosomes/lysosomes (eGFP-Rab7) (C), and lysosomes (eGFP-Lamp1) (D) in enterocytes. Arrowheads: colocalization; n = 3–4, with 3–12 fish per n. APOA-I, apolipoprotein A-I; hAPOA-I, human APOA-I; L, intestinal lumen; N, nucleus. |
|
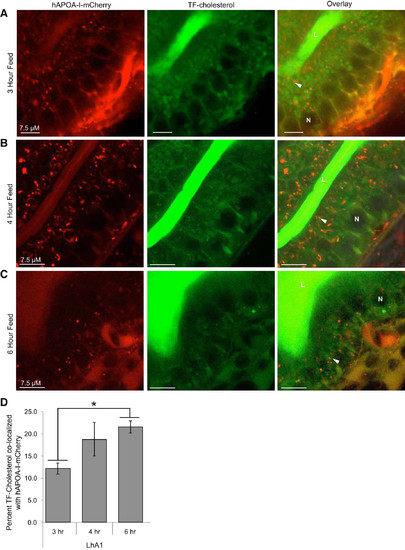
hAPOA-I-mCherry colocalizes with dietary TF-cholesterol in enterocytes of tg(lfabp10:hAPOA-I-mCherry) larvae. Representative images showing hAPOA-I-mCherry and TF-cholesterol colocalization in enterocytes after 3 h (A), 4 h (B), and 6 h (C) of 5% egg yolk feed. Arrowheads: colocalization; n = 3–4, with 3–9 fish per n. A greater percent of TF-cholesterol colocalizes with hAPOA-I-mCherry at 6 h into feeding than at 3 h (means ± SE, 1-way ANOVA of tg(lfabp10:hAPOA-I-mCherry) groups, *P < 0.05) (D). APOA-I, apolipoprotein A-I; hAPOA-I, human APOA-I; L, intestinal lumen; N, nucleus; TF-cholesterol, TopFluor-cholesterol. |