- Title
-
To transduce a zebra finch: interrogating behavioral mechanisms in a model system for speech
- Authors
- Heston, J.B., White, S.A.
- Source
- Full text @ J. Comp. Physiol. A
|
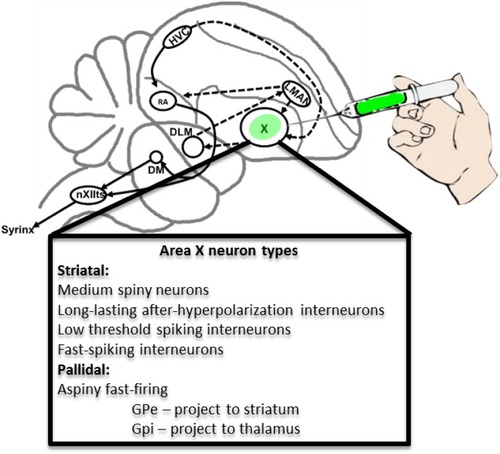
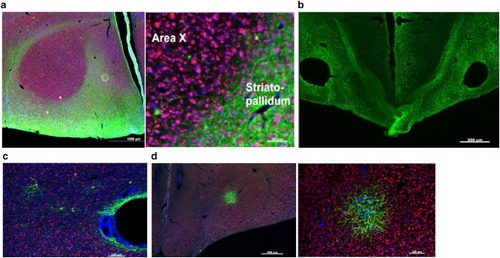
Song control circuitry including neuronal phenotypes of striato-pallidal Area |
|
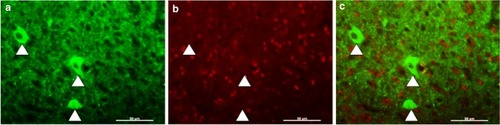
FoxP2 is not co-expressed in pallidal neurons labeled by anti-Lant6 antibody. |
|
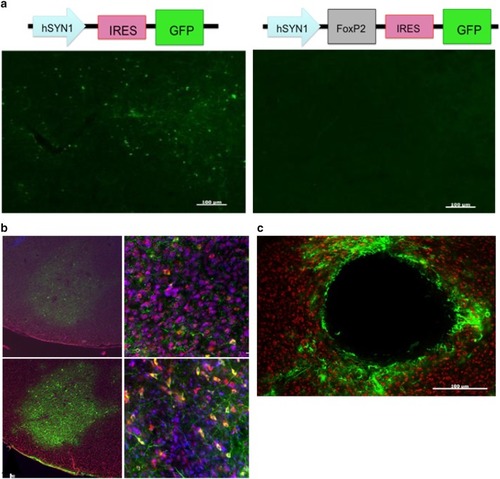
Characterization of three lentiviruses obtained from the UCLA viral vector core for their ability to transduce Area X neurons. |
|
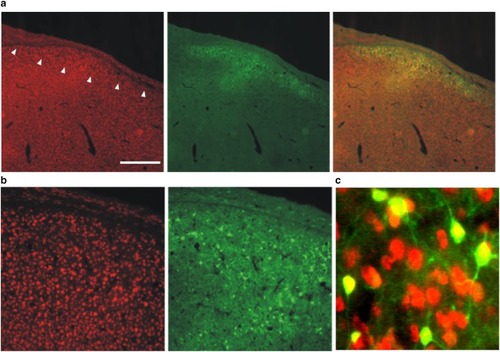
AAV hSyn1 GFP transduces HVC neurons. Photomicrographs show NeuN-positive neurons ( |
|
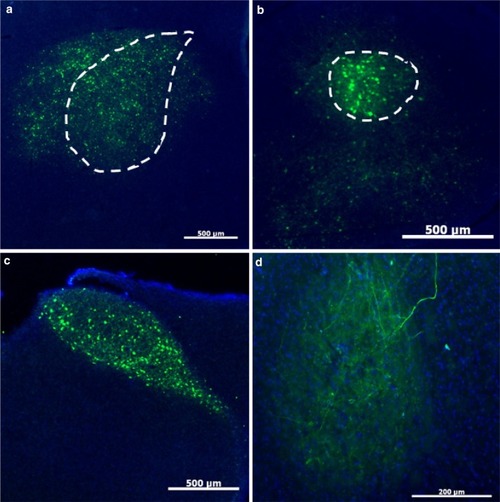
Characterization of four AAVs obtained from the U Penn viral vector core for their ability to transduce Area X neurons. In |
|
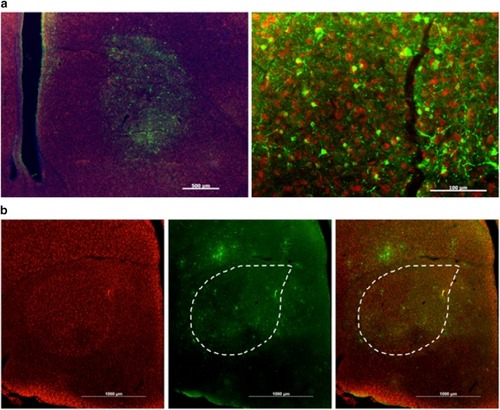
U Penn AAV2/1-CB7-GFP retrogradely infects at least one, if not more, Area X afferent nuclei. |
|
Virovek AAV2/5 is effective in transducing Area X neurons, whereas AAV2/9 is not. |
|
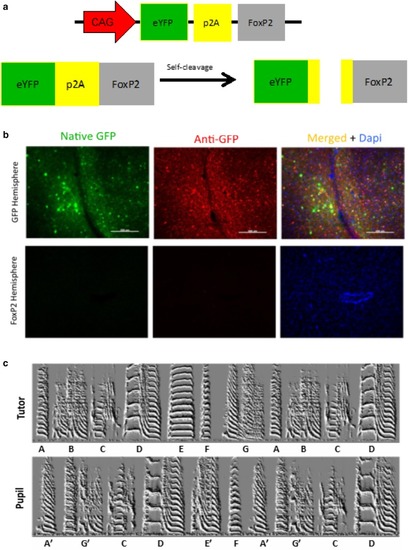
A p2A sequence is ineffective in expressing either GFP or FoxP2. |
|
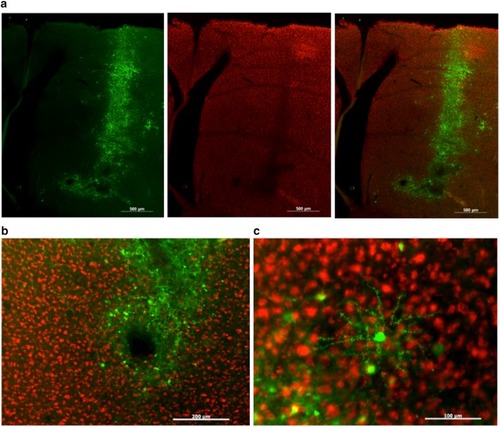
HSV effectively transfects Area X but can cause neurotoxicity. |
|
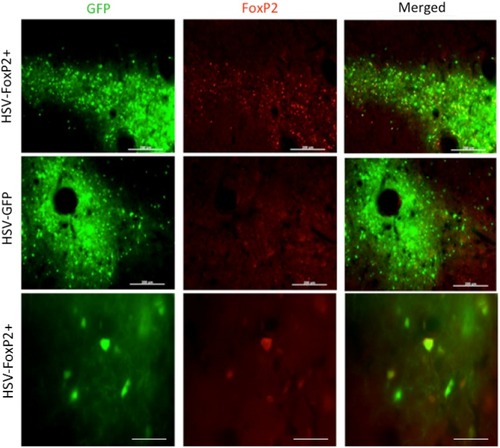
HSV is effective in overexpressing FoxP2. Photomicrographs from a bird that was injected with HSV-FoxP2+ in Area X of one hemisphere ( |
|
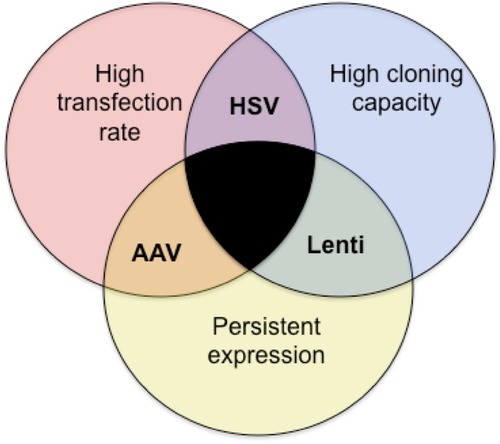
Venn diagram highlights the strengths and limitations of each virus type as they relate to specific requirements necessary for a given scientific application. Those requirements are (1) a high rate of transfection, (2) a high cloning capacity and, (3) persistent expression. Each viral type tested here fulfills only two requirements |