- Title
-
Influenza A Virus Infection Damages Zebrafish Skeletal Muscle and Exacerbates Disease in Zebrafish Modeling Duchenne Muscular Dystrophy
- Authors
- Goody, M., Jurczyszak, D., Kim, C., Henry, C.
- Source
- Full text @ PLoS Curr.
|
Human IAV infects zebrafish muscle cells and causes muscle fiber damage All embryo images are side mounts, dorsal top, anterior left. Panels A-D are phalloidin stained to visualize F-actin. White arrowheads denote retracted fibers. (A) PBS-injected control at 24 hpi (3 dpf). (B) PBS-injected control at 48 hpi (4 dpf). (C) IAV-infected embryo at 24 hpi. (D) IAV-infected embryo at 48 hpi. (E) Quantification of the proportion of muscle segments per embryo with damaged fibers in IAV-infected embryos over developmental time. Fiber damage is more frequently observed at 48 hpi than at 24 hpi. (F) Spatial location of damaged fibers along the anterior-posterior axis of IAV-infected embryos at 24 hpi. The frequency of damaged fibers peaks in segments 5-9, which is in the anterior of the fish near the Duct of Cuvier (the site of injection). (G) PBS-injected control at 24 hpi (3 dpf). (H) NS1-GFP-injected zebrafish at 24 hpi. Note the punctate green fluorescence in infected cells throughout the body. (I) NS1-GFP-injected zebrafish at 24 hpi. Higher magnification view of a GFP-positive, infected muscle fiber. (I1) Merged panel of NS1-GFP and brightfield images. PHENOTYPE:
|
|
IAV infection compromises the sarcolemma All embryo images are side mounts, dorsal top, anterior left. (A) Tg(fli1:GFP) zebrafish embryo with labeled endothelial cells (green) DC-injected with PBS plus EBD (red). EBD remains in the vasculature. White arrowheads point to EBD in an intersomitic vessel (top left), the dorsal aorta (middle), and the caudal vein (bottom right). (B-C) Wild-type zebrafish injected with PBS plus EBD. (B) Cropped EBD panel. (B1) Cropped EBD and brightfield panels merged. (C) EBD panel. Note that muscle fibers are impermeable to EBD in PBS-injected zebrafish. (D) Tg(fli1:GFP) zebrafish embryo DC-injected with IAV plus EBD (red). EBD leaked out of the vasculature and penetrated muscle fibers (black arrowheads). (E-F) Wild-type zebrafish injected with IAV plus EBD. (E) Cropped EBD panel. (E1) Cropped EBD and brightfield panels merged. (F) EBD panel. Note the uptake of EBD by long (black arrowheads) and retracted fibers (white arrowheads) indicative of sarcolemma damage. EXPRESSION / LABELING:
PHENOTYPE:
|
|
IAV infection disrupts muscle fiber-ECM adhesion All embryo images are side mounts, dorsal top, anterior left of zebrafish at 24 hpi. Lettered panels show phalloidin staining for F-actin in red. Panels numbered 1 show immunohistochemistry for beta-Dystroglycan, Dystrophin, or Paxillin proteins in green. Panels numbered 2 are merged images of phalloidin and antibody staining. (A-A2) Phalloidin and beta-Dystroglycan staining in a PBS-injected zebrafish. White arrow in A1 denotes MTJ localized beta-Dystroglycan and white arrowhead in A1 points to neuromuscular junction localized beta-Dystroglycan. (B-B2) Phalloidin and beta-Dystroglycan staining in an IAV-injected zebrafish. White arrow in B points to a retracted fiber and white arrowheads in B1-2 highlight beta-Dystroglycan staining at the unattached end of a retracted fiber. (C-C2) Phalloidin and Dystrophin staining in an IAV-injected zebrafish. White arrow in C points to a retracted fiber and white arrowheads in C1-2 highlight Dystrophin staining at the unattached end of a retracted fiber. (D-D2) Phalloidin and Paxillin staining in an IAV-injected zebrafish. White arrow in D points to a retracted fiber and white arrowheads in D1-2 highlight Paxillin staining at the unattached end of a retracted fiber. These results showing that some retracted fibers retain the localization of ECM adhesion proteins suggest that muscle fibers-ECM adhesion can be disrupted external to the sarcolemma. EXPRESSION / LABELING:
PHENOTYPE:
|
|
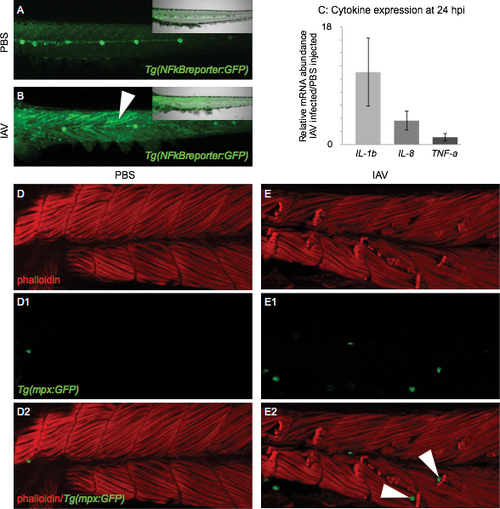
Molecular and cellular markers of inflammation are present in the muscle tissue of IAV-infected zebrafish All embryo images are side mounts, dorsal top, anterior left of zebrafish at 24 hpi. (A) PBS-injected Tg(NFkB:GFP) zebrafish embryo which expresses GFP in cells where NFkB transcription factor-dependent gene expression is occurring. NFkB signaling is active in the lateral line system, but not in muscle cells. Inset panel is a merge of fluorescence and brightfield imaging. (B) IAV-injected Tg(NFkB:GFP) zebrafish embryo. NFkB-dependent gene transcription is turned on in muscle cells in response to IAV infection. Inset panel is a merge of fluorescence and brightfield images. (C) Quantification of pro-inflammatory cytokine mRNA expression in IAV-infected zebrafish compared to PBS-injected zebrafish at 24 hpi. Expression of interleukin 1, beta (11.1 +/- 5.2-fold increase; 3 biological replicates; 3 independent experiments) and interleukin 8 (3.7 +/- 1.4-fold increase; 3 biological replicates; 3 independent experiments) increases in response to IAV while tumor necrosis factor a expression remains unchanged (1.1 +/- 0.5-fold increase; 2 biological replicates; 2 independent experiments). (D-E2) Lettered panels show phalloidin staining (red), panels numbered 1 show GFP-positive neutrophils, and panels numbered 2 are merged. (D-D2) PBS-injected Tg(mpx:GFP) zebrafish. Note that not many neutrophils are present in muscle tissue. (E-E2) IAV-infected Tg(mpx:GFP) zebrafish. Note the retracted muscle fibers and the infiltration of muscle tissue by neutrophils. White arrowheads in E2 point to neutrophils localized to the unanchored ends of retracted muscle fibers. EXPRESSION / LABELING:
PHENOTYPE:
|
|
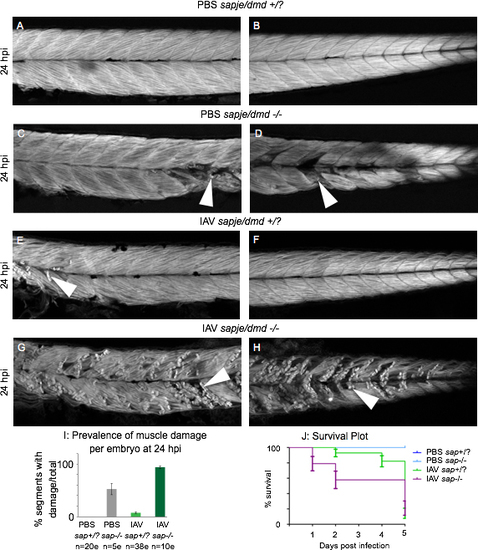
Muscle damage caused by IAV infection is exacerbated in the zebrafish model of DMD All embryo images are side mounts, dorsal top, anterior left of zebrafish at 24 hpi stained with phalloidin to visualize F-actin. White arrowheads point to foci of muscle damage. (A) Anterior muscle segments of a PBS-injected wild-type sibling embryo. (B) Posterior muscle segments of a PBS-injected wild-type sibling embryo. Note the lack of muscle damage present. (C) Anterior muscle segments of a PBS-injected sapje/dmd mutant embryo. (D) Posterior muscle segments of a PBS-injected sapje/dmd mutant embryo. Note that certain muscle segments in the anterior and the posterior have foci of muscle damage. (E) Anterior muscle segments of an IAV-injected wild-type sibling embryo. (F) Posterior muscle segments of an IAV-injected wild-type sibling embryo. Note the damaged fibers in the anterior muscle segments. (G) Anterior muscle segments of an IAV-injected sapje/dmd mutant embryo. (H) Posterior muscle segments of an IAV-injected sapje/dmd mutant embryo. Note the presence of damaged fibers in every imaged muscle segment of this embryo. (I) Quantification of the average number of muscle segments with damaged fibers per embryo at 24 hpi. Note that the prevalence of fiber damage in IAV-infected sapje/dmd mutants is more than would be predicted from adding the prevalence of damaged fibers of IAV-infected zebrafish and sapje/dmd mutants together. (J) Plot tracking survival for 5 days post injection. All PBS-injected wild-type siblings and sapje/dmd mutants lived for 5 dpi (blue lines). Most mortalities were observed in IAV-infected wild-type siblings between 4 and 5 dpi (green line). IAV-infected sapje/dmd mutants succumbed to the infection earlier than their wild-type siblings with more mortalities occurring on the first and second days post infection. Survival curves from individual experiments representative of three replicates. |