- Title
-
Fate predetermination of cardiac myocytes during zebrafish heart regeneration
- Authors
- Tekeli, I., Garcia-Puig, A., Notari, M., García-Pastor, C., Aujard, I., Jullien, L., Raya, A.
- Source
- Full text @ Open Biol.
|
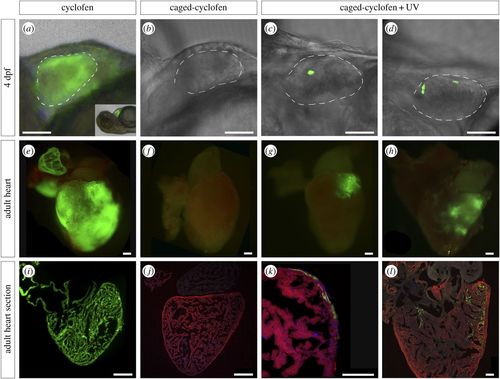
Genetic labelling of zebrafish CMs using a UV-inducible Cre/lox recombination system. Double transgenic embryos (Tg(myl7:Cre-Ert2)+/− × Tg(myl7:LnL-EGFP)+/−) were treated at 2 dpf with (a) uncaged cyclofen (n = ∼100), (b) caged-cyclofen without UV exposure (n = ∼100) and (c,d) caged-cyclofen followed by UV exposure (n = ∼100). One fish tank per condition (approx. 40 larvae) was raised to adulthood. Cyclofen works as a tamoxifen analogue and induces recombination in CMs as observed by GFP expression at 4 dpf (a) and at adult stage (e,i). (b,f,j) Caged-cyclofen cannot induce recombination unless it is uncaged by UV exposure (c,d,g,h,k,l). Adult heart sections were processed for immunofluorescence with antibodies against MF20 and GFP. Nuclei were counterstained with DAPI. Scale bars: (a–d) 150 µm, (e–j) 250 µm, (k–l) 100 µm. Dashed lines in (a–d) define the ventricle perimeter. |
|
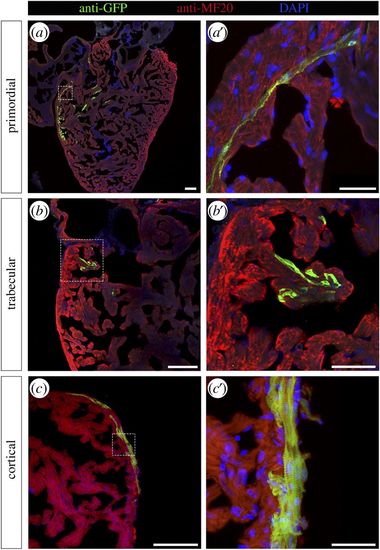
Genetically labelled CMs are found in all three myocardium layers. Adult zebrafish hearts labelled at 2 dpf were sectioned and processed for immunofluorescence with antibodies against MF20 and GFP. GFP-labelled CMs were identified in (a,a′) the primordial layer, (b,b′) the trabecular layer and (c,c′) the cortical layer. Nuclei were counterstained with DAPI. Scale bars: (a–c) 100 µm, (a′–c′) 25 µm. Dashed lines in (a–c) define the magnification areas shown in (a′–c′), respectively. |
|
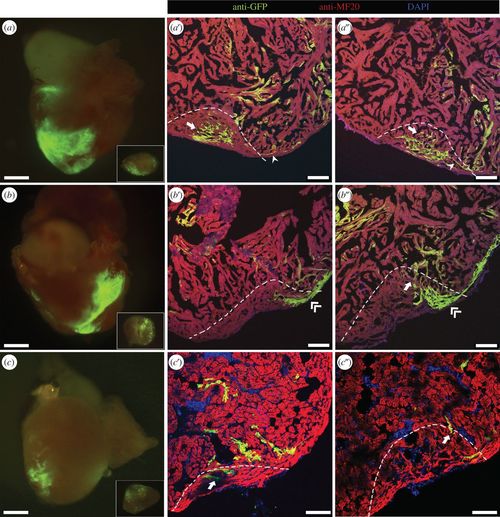
Cell fate of labelled CMs during regeneration. Three-month-old adult zebrafish labelled at 2 dpf were subjected to ventricular amputation, and after 30 dpa the hearts were collected. (a–c) Epifluorescence images of representative hearts where the amputation plane passed through GFP-positive areas. The excised portion of tissue is shown in inset. (a′–c″) Collected hearts were sectioned, and processed for immunofluorescence with antibodies against MF20 and GFP. (a′,a″) Different sections of the regenerated myocardium of the heart shown in (a), where primordial (arrowhead) and trabecular (arrow) labelled CMs were observed. (b′,b″) Different sections of the regenerated myocardium of the heart shown in (b), where trabecular (arrow) and cortical (double-arrowhead) GFP-positive CMs were detected. (c′,c″) Different sections of the regenerated myocardium of the heart shown in (c), where trabecular (arrow) GFP-positive CMs were detected. The amputation plane is indicated by dashed lines. Nuclei were counterstained with DAPI. Scale bars: 500 µm in whole-mount hearts; 100 µm in sections. |
|
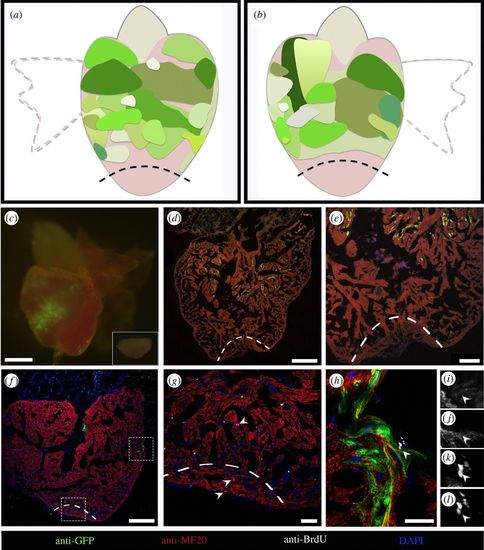
CMs distant from the amputation site do not contribute to the regenerated tissue. (a,b) Diagram summarizing the size and location of GFP-positive areas from different hearts which did not contribute to regeneration (n = 15). (a) The dorsal view of a zebrafish heart and (b) the ventral view. (c) Epifluorescence image of a representative heart where the amputation plane did not pass through the GFP-positive area. The excised portion of tissue is shown in inset. (d,e) Collected hearts were sectioned, and processed for immunofluorescence with antibodies against MHC and GFP. GFP-labelled CMs are absent in the regenerated area. (f–h) Sections processed for immunofluorescence with antibodies against BrdU, GFP and MHC showing the proliferating CMs (arrowheads) in the injury site (g) and in the GFP-positive area (h). Single image channels of the BrdU-positive GFP-positive CM (arrowhead) shown in (h), where (i) is GFP, (j) is MHC, (k) is DAPI and (l) is BrdU. Magnification images of lower and upper dashed rectangles in (f) are shown in (g) and (h), respectively. The amputation plane is indicated by dashed lines. Nuclei were counterstained with DAPI. Scale bars: (c) 500 µm, (d,f) 250 µm, (e) 100 µm, (g) 40 µm and (h)15 µm. |
|
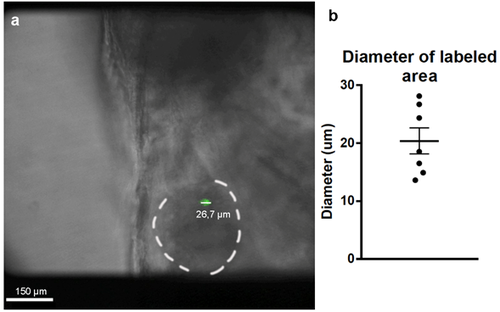
Diameters of the labeled areas in 4dpf zebrafish hearts. (a) Images of the labeled hearts were taken at 4 dpf and the diameters or the GFP-labeled areas were measured using ImageJ. (b) Measured diameters indicated that these labeled areas corresponded to one or two cardiomyocytes (n=7). Mean ± SEM. |
|
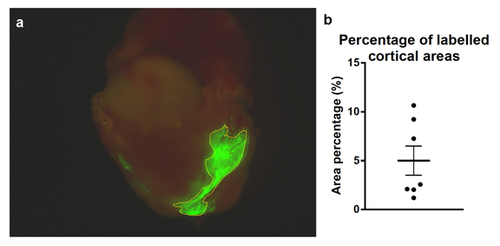
Labeled cortical area size. (a) Percentages of the cortical areas were calculated from the images of whole-mount hearts by using ImageJ. (b) Individual values obtained for 7 labeled cortical areas. Calculated areas ranged from 1.2% to 11%. Mean ± SEM. |
|
Labeled CMs during heart regeneration. 2dpf labeled zebrafish larvae were grown to adulthood (3-months-old) and subjected to ventricular amputation. Regenerating hearts were collected after 30dpa. 19 of the analyzed hearts had clones which did not contribute to the regenerated area. The ventral (a-s) and the dorsal (a'-s') view of each of the 19 hearts at 30dpa are shown. (a''-s'') Excised portion of the tissue in the amputation procedure. dpa, days post amputation. Scale bar: 500µm |
|
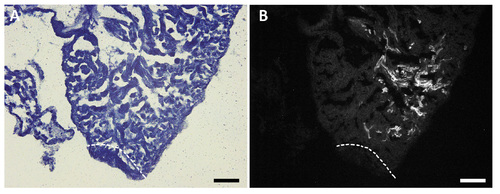
Labeled CMs close to the injury site. Sections processed for Sudan black staining showing the closest labeled cardiomyocytes to the injury site (a,b) in which GFP-positive cardiomyocytes did not contribute to the regenerated tissue. (a) Bright field image and (b) GFP positive clone. This heart corresponds to the regenerated heart in the panel q and q' from the Supplementary figure S4. The amputation plane is indicated by dashed lines. Scale bars: 250μm |