- Title
-
Chemokine Signaling and the Regulation of Bidirectional Leukocyte Migration in Interstitial Tissues
- Authors
- Powell, D., Tauzin, S., Hind, L.E., Deng, Q., Beebe, D.J., Huttenlocher, A.
- Source
- Full text @ Cell Rep.
|
Cxcr1 Is Required for Neutrophil Recruitment to Tissue Wounds, whereas Cxcr2 Is Required for Neutrophil Resolution (A) Sudan black staining of 3 days post-fertilization (dpf) larvae at 1, 3, and 6 hr post-wound (hpw) reveals Cxcr1−/− larvae recruit less neutrophils to tail-transection compared to Cxcr1+/− control. (B) Quantification of neutrophils caudal to the notochord (n = 16 heterozygote [het] and 19 mutant larvae [1 hpw], 18 het and 18 mutant [3 hpw], and 25 het and 21 mutant [6 hpw]). (C) Quantification of total neutrophils in 3 dpf Cxcr1+/− and Cxcr1−/− larvae (n = 19 het and 23 mutant). (D) Sudan black staining of 3 dpf larvae at 3 and 6 hpw show more neutrophils at the wound microenvironment in Cxcr2−/− embryos than WT. (E) Quantification of neutrophils caudal to the notochord in Cxcr2−/− and WT (n = 70 WT and 46 mutant [3 hpw] and 83 WT and 54 mutant [6 hpw]). (F) Total neutrophil number is higher in Cxcr2−/− than WT (n = 48 WT and 40 mutant larvae). Error bars represent SE. ∗p < 0.05. PHENOTYPE:
|
|
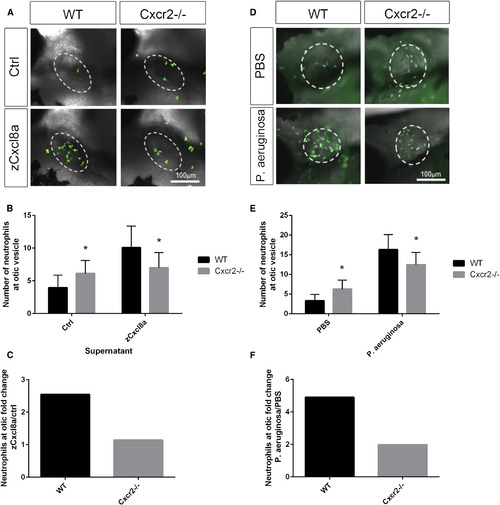
Cxcr2 Is Required for Neutrophil Response to Cxcl8a and Pseudomonas aeruginosa Infection (A) Representative imaging of the otic vesicle (white dotted outline) 2 hr after injection with supernatant from HEK cells overexpressing zebrafish Cxcl8a or control supernatant. Supernatant was injected into the otic vesicle of 3 dpf Tg(mpx:Dendra) WT or Cxcr2−/− larvae (neutrophils in green). (B) Number of neutrophils in the otic vesicle of Cxcl8a or control supernatant injected larvae (n = 59 WT and 58 mutant [Ctrl] and 56 WT and 54 mutant [zCxcl8a]). (C) Neutrophil fold change response to Cxcl8a over control supernatant. (D) Representative imaging of the otic vesicle (white dotted outline) 2 hr after injection with P. aeruginosa in WT or Cxcr2 mutant Tg(mpx:Dendra) larvae. (E) Number of neutrophils in the otic vesicle of P. aeruginosa or vehicle-injected larvae (n = 62 WT and 73 mutant [PBS] and 76 WT and 76 mutant [P. aeruginosa]). (F) Neutrophil fold change response to P. aeruginosa over PBS control. ∗p < 0.05. Error bars represent SE. PHENOTYPE:
|
|
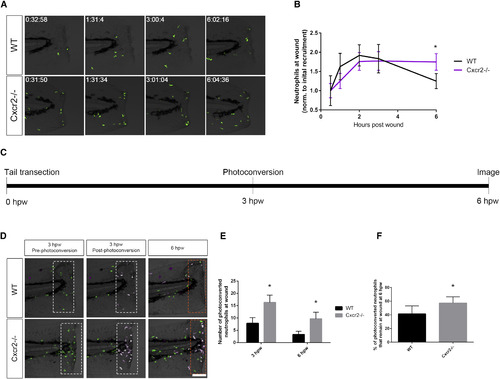
Neutrophil Reverse Migration Is Impaired in cxcr2 Mutants (A) Time-lapse imaging of Tg(mpx:Dendra) WT and Cxcr2−/− tail fins after wounding. (B) Quantification of average neutrophil presence at wound normalized to the average initial neutrophil recruitment to wound at 30 min post-wound (n = 11 WT and 14 mutant; one representative experiment). WT larvae exhibit neutrophil recruitment to wound peaking at 2 hpw with resolution back to 30 min post-wound levels by 6 hpw, whereas Cxcr2−/− larvae maintain high levels of neutrophil infiltration in the wound microenvironment at 6 hpw. (C) Schematic of photoconversion of Cxcr2−/− neutrophils at wound. Tg(mpx:Dendra) Cxcr2−/− larvae were wounded at 3 dpf. Photoconversion of Dendra+ neutrophils within the wounded tail fin (white dotted outlines in D) was performed at 3 hpw, and neutrophil reverse migration was assessed at 6 hpw. (D) Neutrophils pre- and post-photoconversion at 3 hpw and at 6 hpw were imaged in WT and Cxcr2−/−. (E) Quantification of photoconverted neutrophils in the wound microenvironment (magenta cells in dotted boxes, D; n = 25 WT and 30 mutants). cxcr2−/− wounds contained a higher level of photoconverted cells at 3 and 6 hpw. (F) Percent of photoconverted cells that remained at the wound at 6 hpw was calculated. Cxcr2−/− neutrophils remain at the wound at a higher frequency than WT neutrophils. ∗p < 0.05. Error bars represent SE. PHENOTYPE:
|
|
Cxcr2 Inhibitor SB225002 Blocks Neutrophil Reverse Migration (A) Schematic of photoconversion of DMSO- or SB225002-treated larvae. Tg(mpx:Dendra) were wounded with tail transection at 3 dpf. DMSO or SB225002 was applied at 2 hpw. Photoconversion of neutrophils within the wounded fin (white dotted box in D) was performed at 3 hpw, and neutrophil reverse migration was assessed at 6 hpw. (B) Sudan black staining at 6 hpw of WT larvae treated with DMSO or Cxcr2 inhibitor SB225002. Larvae were wounded at 3 dpf and treated with drug or vehicle control at 2 hpw. (C) Quantification of neutrophils caudal to the notochord at 6 hpw (n = 20 DMSO larvae and 18 SB225002). (D) Neutrophils pre- and post-photoconversion at 3 hpw and at 6 hpw were imaged in DMSO- or SB225002-treated larvae. (E) Quantification of photoconverted neutrophils in the wound microenvironment (magenta cells in dotted boxes, D). SB225002-treated larvae contained a higher level of photoconverted cells within the tail fin at 3 and 6 hpw (n = 21 larvae, each). (F) Percent of photoconverted cells that remained at the wound at 6 hpw was calculated. SB225002-treated neutrophils remain at the wound at a higher frequency than in DMSO control. ∗p < 0.05. Error bars represent SE. PHENOTYPE:
|
|
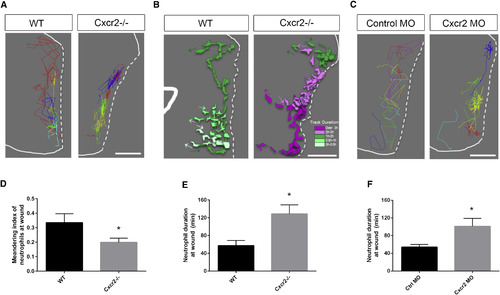
Interstitial Behavior in the Wound Microenvironment Is Altered in cxcr2-Deficient Zebrafish (A) Representative image of neutrophil tracks within the tail fin of WT or Cxcr2−/− larvae. Each color track represents an individual neutrophil (white dotted line traces wound edge). (B) Representative tracks of neutrophil morphology at the wound in WT or Cxcr2−/− larvae. Individual neutrophils are color coded to represent duration at the wound (see legend), with each colored track representing one neutrophil over the duration of its time at the wound. (C) Representative neutrophil tracks at the wound of WT or cxcr2 morphant larvae. (D) Average meandering index of neutrophils at the wound in WT is greater than in Cxcr2−/− larvae (n = 20, each). (E and F) Average neutrophil duration at the wound is greater (E) in Cxcr2−/− compared to WT and (F) in cxcr2 MO compared to WT (n = 20, each). ∗p < 0.05. Error bars represent SE. PHENOTYPE:
|
|
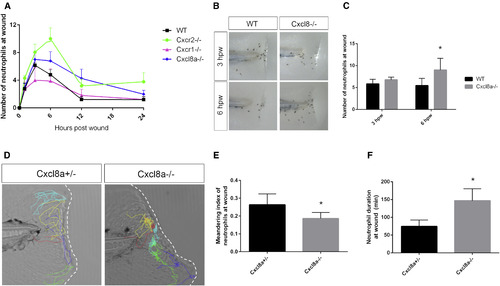
Cxcl8a Is Required for Neutrophil Reverse Migration from the Wound (A) Average number of neutrophils at the wound is compared in WT, cxcr1−/−, cxcr2−/−, and cxcl8a−/− larvae (n = 13–32 larvae per time point/condition). (B) Sudan black staining of neutrophils at 3 and 6 hpw in WT and cxcl8a−/−. (C) Quantification of neutrophils at the wound shows more neutrophils at 6 hpw in cxcl8a−/− compared to heterozygote siblings (n = 74 WT and 75 mutants [3 hpw] and 91 WT and 70 mutants [6 hpw]). (D) Representative tracks of neutrophils at the wound in cxcl8a+/− and cxcl8a−/− larvae. (E) Neutrophil duration at the wound is greater in cxcl8a−/− than in heterozygotes (n = 17 het and 15 mutant larvae). (F) Meandering index is decreased in cxcl8a−/− compared to het siblings. ∗p < 0.05. Error bars represent SE. PHENOTYPE:
|
|
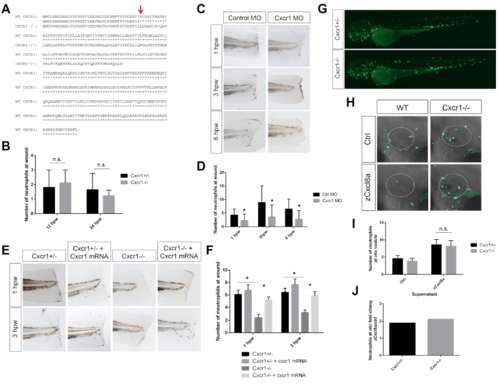
Cxcr1 morpholino and mRNA rescue, related to Figure 1 (A) Amino acid sequence of Cxcr1 TALEN-generated mutation. Red arrow indicates mutated residue. (B) Quantification of neutrophils at the wound at 12 and 24 hpw are similar in Cxcr1-/- compared to Cxcr1+/- siblings (n= 12hpw 15,9; 24hpw 22,9). (C) Sudan black staining of neutrophils in Control MO and Cxcr1 MO injected larvae at 1-6 hpw. (D) Quantification of neutrophils at the wound reveals decreased recruitment in Cxcr1 morphants (n= 1hpw 21, 15; 3hpw 24, 14; 6hpw 19,14, ctrl/Cxcr1 MO respectively). (E) Sudan black staining post-wound of Cxcr1 het and mutant larvae injected with cxcr1 mRNA. (F) Quantification of neutrophils at the wound shows cxcr1 mRNA rescues neutrophil recruitment in Cxcr1-/- (n=1hpw 17, 25, 12, 31; 3hpw 17, 24, 11, 26). (G) Representative images of whole Cxcr1+/- and Cxcr1-/- sibling larvae at 3 dpf expressing Tg(mpx:gfp) to mark neutrophils. (H) Representative imaging of the otic vesicle (white dotted outline) 2 hours after injection with supernatant from HEK cells overexpressing zebrafish Cxcl8a or control supernatant. Supernatant was injected into the otic vesicle of 3 dpf Tg(mpx:gfp) WT or Cxcr1-/- larvae (neutrophils in green). (I) Number of neutrophils in the otic vesicle of Cxcl8a or control supernatant injected larvae (n= ctrl 35, 35; Cxcl8 63, 53). (J) Neutrophil fold change response to Cxcl8a over control supernatant. *p<0.05, n.s.=not significant. |
|
Neutrophil motility and behavior at wound is normal in Cxcr1 mutants, related to Figure 1 (A) Representative tracks of neutrophil migration at the wound in Cxcr+/- and -/- larvae. (B) Average duration of neutrophils at the wound is not significantly different in Cxcr1-/- compared to het siblings (n= 13, 18; B and C). (C) Neutrophil meandering index is not significantly different in Cxcr1+/- and -/-. (D) Representative tracks of random neutrophil migration in the head region of Cxcr1+/- and Cxcr1-/- larvae at 3 dpf. (E) Distance migrated of head neutrophils is not significantly different in Cxcr1+/- and -/- larvae (n=7, 11; E and F, 1 representative experiment). (F) Velocity of randomly migrating neutrophils is not significantly different (n.s.). |
|
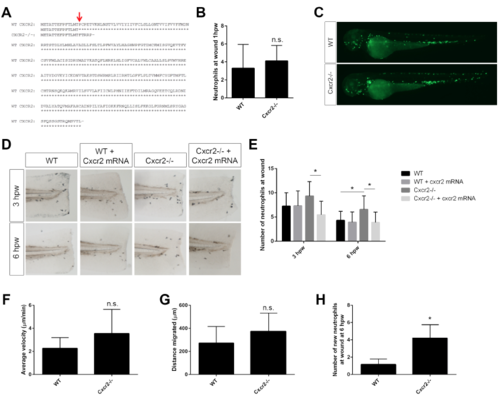
Cxcr2 mutant sequence and rescue, related to Figure 1 (A) Amino acid sequence of Cxcr2 TALEN-generated mutation. Red arrow indicates mutated residue. (B) Initial recruitment of neutrophils to the wound is normal in Cxcr2-/- larvae (n= 49, 43). (C) Representative images of whole WT and Cxcr2-/- larvae at 3 dpf expressing Tg(mpx:Dendra) to visualize neutrophils. (D) Sudan black staining post-wound of WT and Cxcr2-/- larvae injected with cxcr2 mRNA. (E) Quantification of neutrophils at the wound shows cxcr2 mRNA rescues neutrophil resolution in Cxcr2-/- (n= 3hpw 24, 24, 24, 27; 6hpw 30, 33, 29, 28). (F) Velocity and (G) distance migrated of randomly migrating neutrophils is not significantly different in Cxcr2-/- compared to WT (tracks not shown, n= 25, 24). (H) Quantification of new neutrophils recruited to the wound after photo-conversion in Cxcr2-/- is greater at 6 hpw than WT (n= 25, 30). *p<0.05, n.s.= not significant. |
|
Cxcr1 morpholino in Cxcr2 mutants recapitulates Cxcr1 mutant recruitment defect, related to Figure 1 (A) Representative imaging of Tg(mpx:Dendra) neutrophils at the wound in WT or Cxcr2-/- larvae with control or Cxcr1 morpholino. (B) Quantification of neutrophils at the wound demonstrates an increase in Cxcr2-/- which is rescued by Cxcr1 MO at 3 and 6 hpw (n= 3hpw 33, 27, 22, 17; 6hpw 30, 24, 23, 18, respectively). *p<0.05. |
|
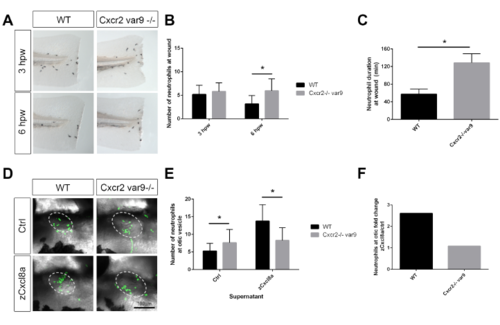
Cxcr2 mutant variant recapitulates neutrophil resolution and migration phenotype, related to Figure 1 (A) Sudan black staining of neutrophils at the wound in a variant Cxcr2-/-. (B) Quantification of neutrophils at wound reveals an increase in neutrophils at 3 and 6 hpw in Cxcr2-/- (n= 3hpw 71, 69; 6hpw 84, 59). (C) Neutrophil duration at wound is increased in Cxcr2-/- variant 9 (n= 20, each). (D) Neutrophil (green) recruitment to otic vesicle injection of zCxcl8 supernatant is decreased in Cxcr2-/- var9 compared to WT. (E) Number of neutrophils in the otic vesicle of Cxcl8a or control supernatant injected larvae (n= ctrl 42, 33; Cxcl8 36, 29). (F) Neutrophil fold change response to Cxcl8a over control supernatant. *p<0.05. |
|
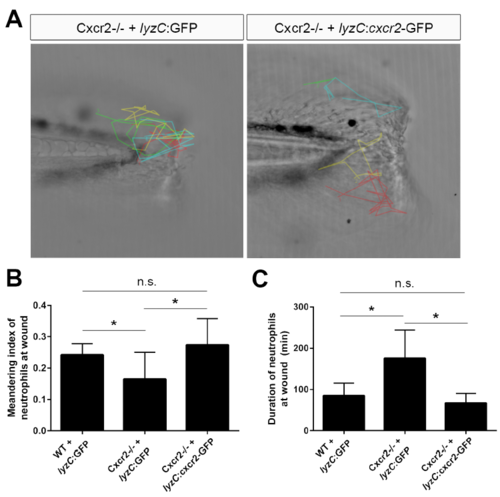
Neutrophil-specific rescue of Cxcr2 rescues neutrophil behavior at wound, related to Figure 5 (A) Cxcr2-GFP was expressed from the neutrophil-specific lyzC promoter in Cxcr2 deficient embryos and neutrophils were tracked following tail-transection. Representative tracks from Cxcr2-/- + lyzC:GFP or lyzC:cxcr2-GFP shown. (B) Average meandering index of neutrophils at the wound in Cxcr2-/- larvae expressing lyzC:cxcr2-GFP is greater compared to lyzC:GFP control and (C) average neutrophil duration at the wound is rescued in Cxcr2-/- + lyzC:cxcr2- GFP to WT levels (n= WT+GFP 15 total cells from 5 larvae, mutant+GFP 35 cells/8 larvae, mutant+Cxcr2 19 cells/7 larvae, 1 representative experiment), *p<0.05. |
|
Cxcl8 mutant sequence, total neutrophils and macrophage recruitment, related to Figure 6 (A) Amino acid sequence of Cxcl8a TALEN-generated mutation. Red arrow indicates mutated residue. Cxcl8a-/- has a 2 a.a. deletion. (B) Representative images of whole Cxcl8a+/- and Cxcl8a-/- sibling larvae at 3 dpf expressing Tg(mpx:gfp) to mark neutrophils. (C) Quantification of total neutrophil number is not significantly different in Cxcl8a-/- compared to heterozygous siblings (n=34, 20). (D) L-plastin antibody staining was performed on Cxcl8a-/- and +/- embryos expressing Tg(mpx:Dendra) at 1, 6, and 20 hpw to label neutrophils (red and green double positive) and macrophages (red only) at the wound. (E) Macrophage numbers at the wound at each time point was not significantly different in Cxcl8a mutants while neutrophil number at the wound (F) was significantly higher at 6 hpw as shown previously (Figure 6B-C, n= 1hpw 16, 10; 6 hpw 12, 12; 20 hpw 14, 5; 1 representative experiment). (G) L-plastin antibody staining was performed on Cxcr2-/- and WT embryos expressing Tg(mpx:Dendra) at 1, 6, and 20 hpw. (H) Macrophage recruitment to the wound was significantly higher in Cxcr2-/- at 6 hpw as was neutrophil presence (I, n= 1hpw 18, 20; 6hpw 23, 25; 20 hpw 20, 21; 1 representative experiment), *p<0.05, n.s.= not significant. |