- Title
-
Gain-of-function defects of astrocytic Kir4.1 channels in children with autism spectrum disorders and epilepsy
- Authors
- Sicca, F., Ambrosini, E., Marchese, M., Sforna, L., Servettini, I., Valvo, G., Brignone, M.S., Lanciotti, A., Moro, F., Grottesi, A., Catacuzzeno, L., Baldini, S., Hasan, S., D'Adamo, M.C., Franciolini, F., Molinari, P., Santorelli, F.M., Pessia, M.
- Source
- Full text @ Sci. Rep.
|
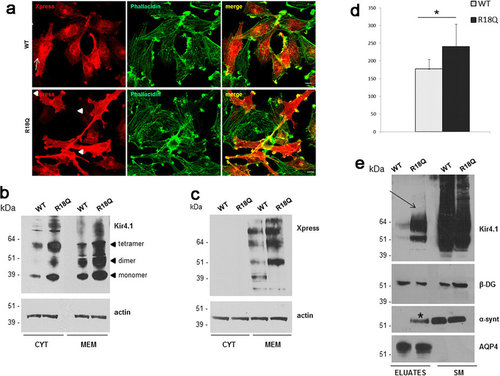
WT and mutated Kir4.1 expression and distribution in U251 astrocytoma cells. (a) Co-immunofluorescence staining of Kir4.1 WT and R18Q expressing cells using Anti-Xpress epitope Ab to stain recombinant Kir4.1 (red) and phallacidin to stain actin filaments (green) shows that WT channels are mostly localized in the cytoplasm, and at plasma membrane in a low percentage of cells (top panels, arrows), while R18Q mutant channels are mainly distributed along cell membranes, filopodia-like structures and cell-cell contacts (bottom panels, arrowheads), and partially co-localizes with actin, in the majority of cells (70%). (b,c) Western Blot (WB) analysis of total membrane and cytosolic protein extracts of astrocytoma cells expressing WT and R18Q Kir4.1, probed with anti Kir4.1 Ab (b) and Anti-Xpress epitope tag Ab (c) revealed that R18Q is more abundantly expressed in the cytoplasm (CYT) and particularly in the total membrane protein fraction (MEM), than the WT protein. Anti-Xpress epitope tag Ab does not detect Kir4.1 protein in the cytoplasm. Arrowheads on the right of panel b highlight the monomeric and oligomeric forms of the Kir4.1 channel. Actin is used as loading control. Molecular weight markers are on the left (kDa). (d) Densitometric analysis of recombinant Kir4.1 bands derived from total membrane protein extracts from WT or R18Q Kir4.1 expressing cells, detected by anti-Xpress Ab and normalized to the corresponding actin value (mean ± SEM, expressed as arbitrary units; *P < 0.05 from three independent experiments). (e) WB of total cell proteins (SM) and enriched fraction of plasma membrane proteins after biotinylation experiments (Eluates) probed with anti-Kir4.1 Ab. Higher amount of R18Q Kir4.1 is expressed at the plasma membrane (arrow) when compared to Kir4.1 WT. Among Kir4.1 associated proteins α-syntrophin (α-synt) is found at plasma membrane of Kir4.1 R18Q mutant expressing cells (asterisk) but not in WT expressing cells. No differences are observed in β-dystroglycan (β-DG) and AQP4 association to astrocytoma plasma membrane. One representative experiment out of two performed with the same results has been shown. |
|
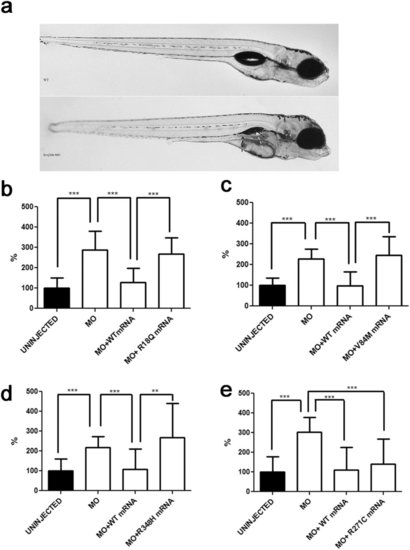
In vivo modelling of Kir4.1 mutations in zebrafish. (a) Transient kcnj10a knockdown zebrafish show macroscopic abnormalities in organ development compared to wild-type (WT). In morphant embryos, the pronephric duct is visible because it is dilated (black arrow); the swim bladder is not visible (white arrow). (b-e) Number of spontaneous tail flicks (registration time 30sec) seen in WT and mutant 30 hpf embryos. Values are expressed as percent of flicks counted in uninjected embryos. Embryos injected with MO (b-e), and with equimolar amount of MO and either R18Q (b), V84M (c), and R348H (d) mRNA show an increased rate of spontaneous contractions, compared to uninjected embryos (b-e), and to either MO+WT (b-e) and MO+R271C (e) mRNA injected embryos. **p < 0.01; ***p < 0.001. PHENOTYPE:
|
|
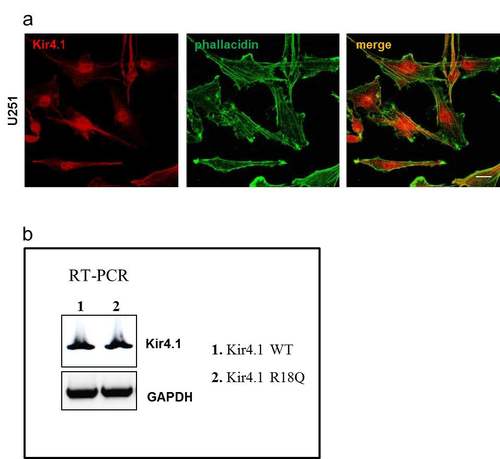
WT and mutated Kir4.1 expression and distribution in U251 astrocytoma cells. (a) Immunofluorescence stainings of U251 astrocytoma cells with anti-Kir4.1 pAb (red) and FITC-conjugated phallacidin (green) to stain actin filaments show that the endogenous Kir4.1 channels are mainly distributed in cytoplasmic perinuclear area and scarcely at plasma membrane. Scale bars: 10 µm. (b) RT-PCR analysis using primers to detect specifically recombinant Kir4.1 mRNA expression (Forward: Xpress epitope/pcDNA 3.1/His, Life technologies; Kir4.1 Reverse: TCAGACATTGCTGATGCGCAC) in stably infected U251 cells reveals no differences between WT (1) and R18Q Kir4.1 (2) expressing cells. GAPDH housekeeping gene normalizes the amount of template used. |
|
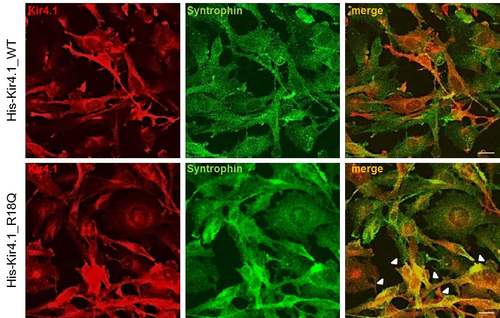
Double immunofluorescence stainings with anti-Kir4.1 pAb (red) and anti-synt mAb (green) in U251 cells expressing WT (upper panels) or R18Q (lower panels) Kir4.1 reveal a partial colocalization of Kir4.1 and syntrophin in the plasma membrane and in the cytoplasm of both astrocytoma cell lines. Compared to Kir4.1 WT expressing cells, a larger number of R18Q+ cells shows colocalization of syntrophin and Kir4.1 in both cytoplasm and plasma membrane (arrowheads). Scale bars: 10 µm. |
|
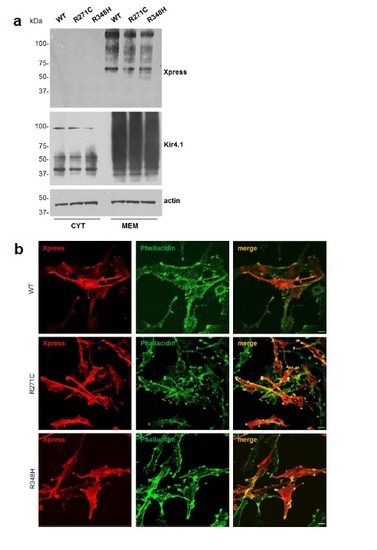
Characterization of astrocytoma cells expressing WT, R271C and R348H Kir4.1 channels. (a) WB analysis of cytosolic (CYT) and membrane (MEM) protein fractions derived from astrocytoma cells stably expressing WT and Kir4.1 mutations R271C and R348H. No significant differences in Kir4.1 expression levels were observed between WT and mutated Kir4.1 expressing cells. Actin is used as internal loading control. Molecular weight markers are on the left (kDa). (b) Co-immunofluorescences of astrocytoma cells expressing WT or mutated channels with anti-Xpress mAb (red) and FITC-conjugated phallacidin (green) show no differences in the distribution of WT, R271C and R348H channels in the cytoplasm and plasma membrane of U251 cells. Scale bar: 10 µm |