- Title
-
Quercetin-4'-O-beta-D-glucopyranoside (QODG) Inhibits Angiogenesis by Suppressing VEGFR2-Mediated Signaling in Zebrafish and Endothelial Cells
- Authors
- Lin, C., Wu, M., and Dong, J.
- Source
- Full text @ PLoS One
|
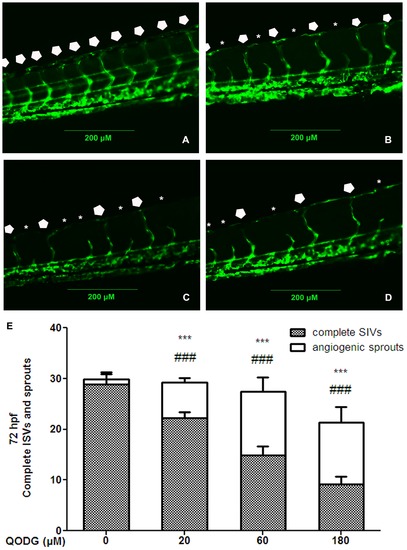
QODG inhibits blood vessel formation in ISVs of zebrafish embryos. (A) Vehicle control: Tg(fli1:EGFP) zebrafish embryos were treated with 0.1% DMSO from 6 hours post fertilization (hpf) to 72 hpf. All intersegmental vessels (ISVs) in the vehicle control group have fully extended to form the dorsal longitudinal anastomotic vessels (DLAVs) at 72 hpf. (B–D) QODG-treated groups: Tg(fli1:EGFP) zebrafish embryos were treated with various concentrations of QODG (20, 60, 180 μM) from 6 hpf to 72 hpf. Pentagons indicate the sites of complete ISVs in zebrafish embryos for all figures, and asterisks indicate the sites of angiogenic sprouts in zebrafish embryos for all figures. (E) Quantitative comparison of blood vessel formation in the vehicle control group and QODG-treated groups. Data are expressed as mean ± SD from three independent experiments. *, the number of complete ISVs in QODG-treated group compared with that in the vehicle control group; ***, P<0.001 vs. vehicle control. #, the number of angiogenic sprouts in QODG-treated group compared with that in the vehicle control group; ###, P<0.001 vs. vehicle control. Scale bars, 200 μm. |
|
QODG inhibits angiogenesis in regenerative caudal fin of zebrafish at 7 days post amputation. (A) Vehicle control: Tg(fli1:EGFP) zebrafish caudal fins were clipped at the mid-fin level, then the fish were allowed to recover in 0.1% DMSO for 7 days post amputation (dpa). (B–D) QODG-treated groups: Tg(fli1:EGFP) zebrafish were allowed to recover in various concentrations of QODG (20, 60, 180 μM) for 7 dpa. Orange arrow indicates the amputation site in caudal fin of adult zebrafish for all figures. (E) Quantitative comparison of the lengths of regenerative vessel and fin in the vehicle control group and QODG-treated groups. Data are expressed as mean ± SD from three independent experiments. *, the length of regenerative vascularized fin tissue in QODG-treated group compared with that in the vehicle control group; ***, P<0.001 vs. vehicle control. #, the length of regenerative nonvascularized fin tissue in QODG-treated group compared with that in the vehicle control group; ###, P<0.001 vs. vehicle control. (F) Quantitative comparison of the vessel densities of regenerative caudal fin in the vehicle control group and QODG-treated groups. Data are expressed as mean ± SD from three independent experiments. *, the vessel density of regenerative caudal fin in QODG-treated group compared with that in the vehicle control group; ***, P<0.001 vs. vehicle control. Scale bars, 200 μm. |
|
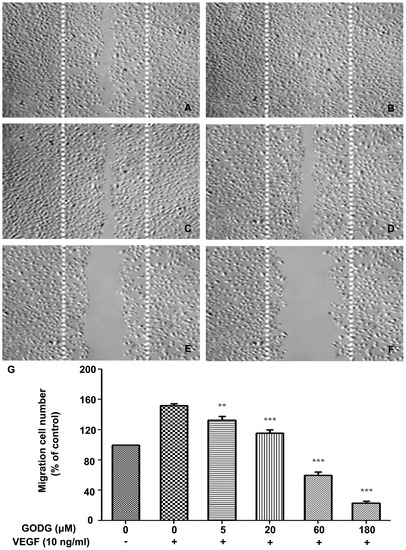
QODG inhibits VEGF–induced chemotactic motility of endothelial cells. QODG inhibited the migration of HUVECs. HUVECs were allowed to grow to full confluence in 6-well plates pre-coated with 0.1% gelatin and then starved with ECGM containing 0.5% FBS to inactivate cell proliferation. After that, cells were wounded with pipette and washed with PBS, then treated with or without VEGF (10 ng/mL) and DMSO (0.1%) or different concentrations of QODG (5, 20, 60, 180 μM) in ECGM containing 0.5% FBS. Images were taken using an inverted microscope (Olympus, Center Valley, PA, USA) (at 100×magnification) after 8 h of incubation, and migrated cells were quantified by manual counting. (A) Migration assay of HUVECs treated with only DMSO (0.1%). (B) Migration assay of HUVECs treated with VEGF (10 ng/mL) and DMSO (0.1%). (C–F) Migration assay of HUVECs treated with VEGF (10 ng/mL) and various concentrations of QODG (5, 20, 60, 180 μM). (G) Quantitative comparison of the numbers of migrated cells in different groups. Cells receiving only DMSO (0.1%) served as a vehicle control. Data are expressed as percentages of the vehicle control (100%) in mean ± SD from three independent experiments. **, P<0.01 vs. VEGF-treated control; ***, P<0.001 vs. VEGF-treated control. Scale bars, 100 μm. |
|
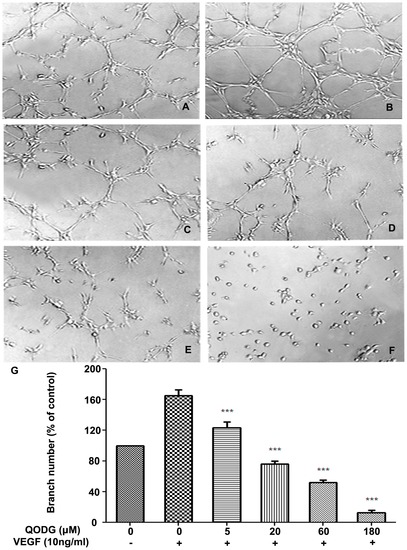
QODG inhibits VEGF-induced capillary structure formation of endothelial cells on Matrigel. QODG inhibited VEGF-induced tube formation of HUVECs. HUVECs were starved with ECGM containing 0.5% FBS, and then treated with DMSO (0.1%) or various concentrations of QODG (5, 20, 60, 180 μM). After that, cells were collected and placed in 24-well plates coated with Matrigel (4×104 cells/well), followed by the activation of VEGF (10 ng/mL). After 6 h of incubation, images of the network-like structures of endothelial cells were taken using an inverted microscope (Olympus, Center Valley, PA, USA) (at 100×magnification), and branching points in different groups were quantified by manual counting. (A) HUVECs cultured on Matrigel were treated with only DMSO (0.1%). (B) HUVECs cultured on Matrigel were treated with VEGF (10 ng/mL) and DMSO (0.1%). (C–F) HUVECs cultured on Matrigel were treated with VEGF (10 ng/mL) and various concentrations of QODG (5, 20, 60, 180 μM). (G) Quantitative comparison of the numbers of branching points in different groups. Cells receiving only DMSO (0.1%) served as a vehicle control. Data are expressed as percentages of the vehicle control (100%) in mean ± SD from three independent experiments. ***, P<0.001 vs. VEGF-treated control. Scale bars, 100 μm. |
|
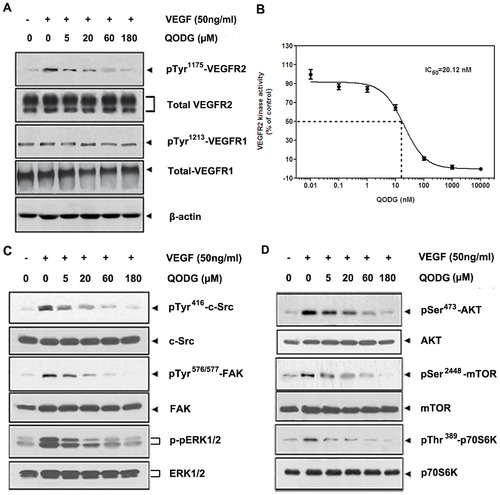
QODG inhibits VEGF-induced phosphorylation of VEGFR2 kinase and VEGFR2-mediated signaling pathway downstream molecules in HUVECs. (A) QODG inhibited VEGF-induced phosphorylation of VEGFR2 in a dose-dependent manner, but phospho-VEGFR1 protein, the total amount of VEGFR1 and VEGFR2 proteins in each sample of cells all remained comparable. After probed with the antibodies anti-VEGFR2, anti-VEGFR1, anti-phospho-VEGFR2 and anti-phospho-VEGFR1, total VEGFR2 and VEGFR1 proteins, and phospho-VEGFR2 and VEGFR1 proteins in different groups were examined by Western blotting analysis. Three independent experiments were performed in triplicates. (B) QODG inhibited VEGFR2 kinase activity. Inhibition of VEGFR2 kinase activity by QODG was analyzed using an in vitro HTScan® VEGF receptor 2 kinase kit (Cell Signaling Technology, Danvers, MA, USA) combined with colorimetric ELISA detection according to the manufacturer′s instructions. The reaction processed with only DMSO (0.1%) served as a vehicle control. Data are expressed as percentages of the vehicle control. Three independent experiments were performed. (C, D) QODG inhibited the activation of VEGFR2-mediated downstream signaling. The activation of c-Src, FAK and ERK (C), AKT, mTOR and p70S6K (D) was suppressed by QODG. After probed with specific antibodies, proteins in different groups were examined by Western blotting analysis. Three independent experiments were performed in triplicates. |
|
QODG potentiates apoptosis in HUVECs in a dose-dependent manner. (A–B) Relative percentages of early apoptotic cells (annexin-V+/PI-) and necrotic or late apoptotic cells (annexin-V+/PI+) were analyzed with one-way ANOVA followed by Tukey′s multiple comparison test. Cells receiving only DMSO (0.1%) served as a vehicle control. Data are expressed as percentages of the vehicle control (100%) in mean ± SD from three independent experiments. (A) The percentages of early apoptotic cells and necrotic or late apoptotic cells increased in a dose-dependent manner when HUVECs were treated without VEGF. #, the percentage of early apoptotic cells (annexin-V+/PI-) in QODG-treated group compared with that in the vehicle control group; ##, P<0.01 vs. vehicle control; ###, P<0.001 vs. vehicle control. *, the percentage of late apoptotic cells (annexin-V+/PI+) in QODG-treated group compared with that in the vehicle control group; ***, P<0.001 vs. vehicle control. (B) The percentages of early apoptotic cells and necrotic or late apoptotic cells increased in a dose-dependent manner when HUVECs were treated with VEGF. #, the percentage of early apoptotic cells (annexin-V+/PI-) in QODG-treated group compared with that in the VEGF-treated control group; ##, P<0.01 vs. VEGF-treated control; ###, P<0.001 vs. VEGF-treated control. *, the percentage of late apoptotic cells (annexin-V+/PI+) in QODG-treated group compared with that in the VEGF-treated control group; *, P<0.05 vs. VEGF-treated control; ***, P<0.001 vs. VEGF-treated control. (C–D) QODG induced caspase-3 activation and the cleavage of PARP from its intact form to its cleaved form. Proteins from HUVECs treated with or without VEGF (10 ng/mL) and DMSO (0.1%) or various concentrations of QODG (5, 20, 60, 180 μM) were analyzed by Western blotting analysis for cleaved caspase-3 and cleaved PARP. |