- Title
-
Rbfox-regulated alternative splicing is critical for zebrafish cardiac and skeletal muscle functions
- Authors
- Gallagher, T.L., Arribere, J.A., Geurts, P.A., Exner, C.R., McDonald, K.L., Dill, K.K., Marr, H.L., Adkar, S.S., Garnett, A.T., Amacher, S.L., and Conboy, J.G.
- Source
- Full text @ Dev. Biol.
|
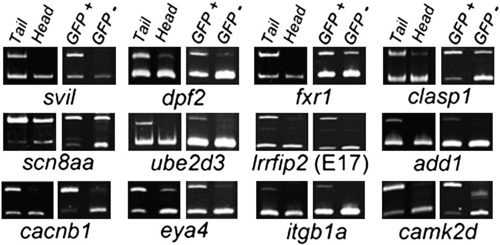
Muscle-enriched splicing of predicted Rbfox-regulated target exons. Semi-quantitative RT-PCR splicing analysis reveals 12 representative alternative exons that are better included in trunk/tail (largely muscle) than head (muscle-poor) fractions at 24 h post fertilization (hpf), as well as in FACS-purified GFP+ muscle versus GFP- cells from actc1b:GFP embryos at 24 hpf. Subsequent analyses show that splicing of 8 of the 12 exons (the top two rows) shown is regulated by rbfox1l and/or rbfox2. Upper product = inclusion isoform; lower product = exclusion isoform. PCR primers and expected band sizes for inclusion and exclusion isoforms are listed in Supplementary Table S1. |
|
Zebrafish rbfox1l and rbfox2 are expressed in muscle cells. rbfox1l (A–G) and rbfox2 (H–N) expression between the 1-somite stage and 42 hpf are shown in dorsal (A, C, D, G–I, N) and lateral (B, E, F, J–M) views. rbfox1l is expressed in adaxial slow muscle precursor cells (arrowhead in A, D), the anterior presomitic mesoderm (PSM; asterisk in D) and formed somites (small arrows in B, D, E), cardiac mesoderm (B, C, E, E2) and pectoral fin (large arrows in F, G), hypaxial body wall (bracket in G), and head musculature (F, G). rbfox2 is expressed more broadly, with enrichment in the PSM and newly formed somites (small arrows in I–K) at early times (H–N). Strong rbfox2 expression is detected in the tail bud (J–L), but myogenic rbfox2 expression decreases as somites mature (K, L). By 36 hpf, rbfox2 transcripts are detected predominantly in the head and pectoral fin (large arrows in M, N). Panel C is a magnified image, in dorsal view, of the boxed region in B. Scale bar = 200 μm for all panels except C (100 μm). EXPRESSION / LABELING:
|
|
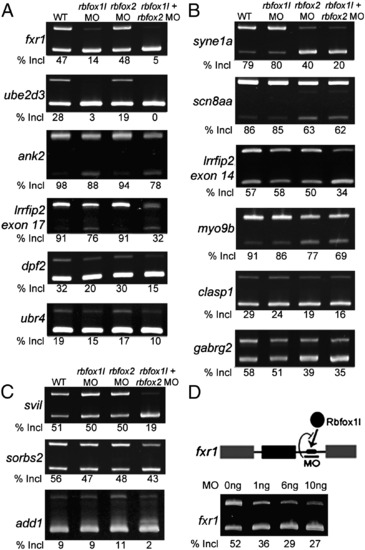
Rbfox knockdown reveals three classes of regulated exons. Semi-quantitative RT-PCR splicing analysis of candidate target exons revealed exon skipping upon knockdown of rbfox1l (A), rbfox2 (B), or both rbfox genes (C). (D) An MO targeting the single Rbfox binding motif in the fxr1 intron reduced exon inclusion to a similar extent as knockdown of rbfox1l expression (A). All results were reproducible in at least 3 experiments (average exon inclusion levels noted below each gel). Alternative exon (black), constitutive exons (gray), Rbfox1l protein (black oval), Rbfox UGCAUG binding motif (black rectangle). In all panels, WT = mock-injected embryos except in D, WT = uninjected embryos, MO = morpholino, E = exon, Incl = inclusion. PCR primers and expected band sizes for inclusion and exclusion isoforms are listed in Supplementary Table S1. |
|
Rbfox1l/Rbfox2 knockdown results in cardiac and skeletal muscle defects. (A, B) In Rbfox double morphants, myotomes are chevron-shaped as in control embryos (lateral view, one myotome is false-colored in each panel). (C–H) Polarized light microscopy reveals that rbfox1l/rbfox2 morphant muscle has delayed birefringence at 30 hpf (compare C with D) that partially recovers by 48 hpf (compare E with F). (G–I) Rbfox1l/Rbfox2 knockdown in myl7-GFP embryos that have GFP + myocardial cells, reveals cardiac muscle defects, with low rbfox1l/rbfox2 MO doses (1.5 ng each) causing ventricular defects and high doses (9 ng each) affecting the morphology of the entire heart. WT = mock-injected; MO = morpholino; a = atrium; v = ventricle. Scale bars = 100 μm (A–B; G–I), 500 μm (C–F). |
|
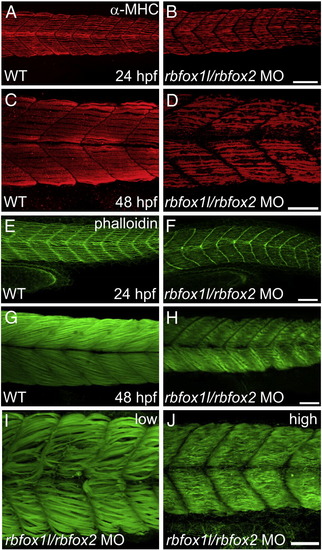
Rbfox1l/rbfox2 knockdown alters myofibril organization. (A–D) Anti-MHC immunohistochemistry at 24 and 48 hpf reveals that slow muscle fibers in rbfox1l/rbfox2 morphants are disorganized compared to normally striated fibers in wildtype muscle (A, C vs. B, D). (E–J) Immunohistochemistry with Alexa-Fluor 488-conjugated phalloidin, which labels F-actin, reveals that slow and fast muscle thin filaments are elongated and highly organized in wildtype embryos at 24 and 48 hpf, respectively (E, G), whereas thin filament structure is severely disrupted in Rbfox1l/rbfox2 morphants (F, H). Defects in myofiber organization (I–J) are less severe at lower rbfox1l/rbfox2 MO doses (1.5 ng each) when compared to higher doses (7 ng each). Doses in B, D, F, and H = 4.5 ng each MO. Scale bars = 50 μm. |
|
Ultra-structural analysis of rbfox1l/rbfox2 morphants reveals disorganized sarcomeres that are reduced in number. Electron micrographs of longitudinal sections of 48 hpf wildtype muscle at 390× and 6000× magnification reveal highly ordered sarcomeres with electron dense Z disks terminating with T-tubules at the sarcoplasmic reticulum (asterisks in C), which contrasts sharply with the dramatic reduction in sarcomere number and organization observed in rbfox1l/rbfox2 morphants at the same magnification (compare A, C with B, D). In the latter, vesicles of the sarcoplasmic reticulum are disorganized and T-tubules are not observed or are unidentifiable at Z-disk boundaries (asterisk in D). Cross sections at 390× and 4000× reveal defects in overall muscle fiber size as well as thick and thin filament arrangement between wildtype embryos and rbfox1l/rbfox2 morphants (E, G vs. F, H). Scale bars = 2.0 μm (A–B, E–F), 0.2 μm (C–D, G–H). PHENOTYPE:
|
|
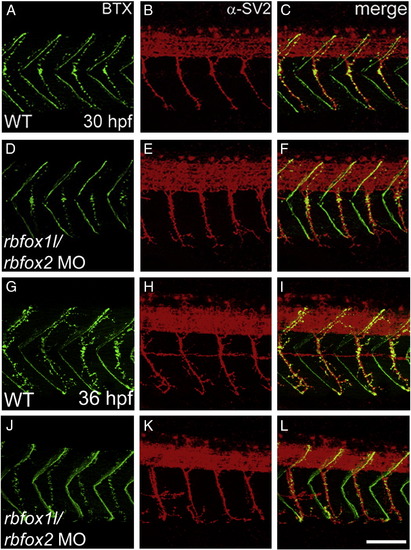
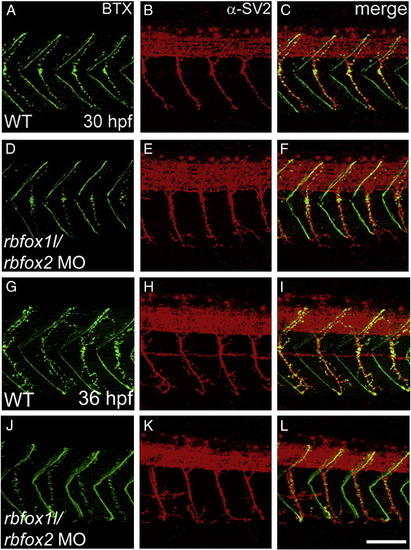
Rbfox1l/rbfox2 knockdown does not alter neuromuscular junction structure. Pre- and post-synaptic neuromuscular junctions at 30 hpf (A–F) and 36 hpf (G–L), stained with anti-SV2 (red) and alpha-bungarotoxin (BTX, green), respectively, appear structurally normal (lateral view, A–C, G–I vs. D–F, J–L). WT = mock-injected; MO = morpholino. Scale bar = 50 μm. PHENOTYPE:
|
|
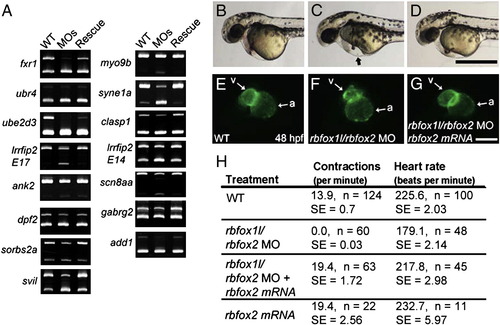
Splicing and physiological defects are rescued with exogenous rbfox2. (A) Splicing analyses of 15 exons that are abnormally spliced upon rbfox1l, rbfox2, or double rbfox1l/rbfox2 knockdown show that normal splicing patterns are restored upon co-injection of MO-resistant rbfox2 mRNA. (B–G) Co-injected rbfox2 mRNA rescued heart-related defects caused by rbfox1l/rbfox2 knockdown. At 48 hpf, rbfox1l/rbfox2 double morphants exhibit pericardial edema (arrow in C, compare to wildtype embryo in B) that was reduced by co-injection with rbfox2 mRNA (D). Ventral views of the same myl7-GFP embryos shown in B–D reveal that ventricle morphology of embryos co-injected with rbfox1l/2 MOs and rbfox2 mRNA (G) are indistinguishable from wild type (E). (H) Functional defects in skeletal muscle and heart in Rbfox double morphants, as measured by muscle contractions at 24 hpf and heart rate at 52 hpf, respectively, are rescued by co-injection of rbfox2 mRNA. WT = mock-injected; MOs = rbfox1l + rbfox2 MO; MO = morpholino; E = exon; a = atrium; v = ventricle; n = number of individuals sampled; SE = standard error. Scale bars = 500 μm (B–D), 100 μm (E–G). |
|
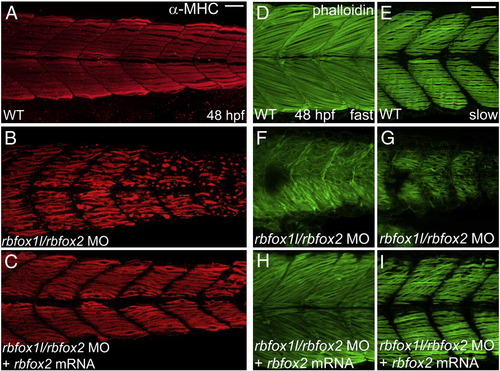
Myofibril defects are rescued with exogenous rbfox2. (A–C) Anti-MHC immunohistochemistry at 48 hpf reveals that slow muscle fiber thick filament morphology in rbfox2 mRNA-injected double morphant embryos closely resembles wild-type morphology and is substantially improved compared to that in embryos injected with rbfox1l/rbfox2 MOs alone, although some wavy fibers persist. (D–I) Similarly, thin filament structure detected using Alexa-Fluor 488-conjugated phalloidin appears elongated and highly organized in both deeper fast muscle and more superficial slow muscle layers of rbfox2 mRNA-injected double morphants when compared to mock-injected wildtype controls and embryos injected with rbfox1l/rbfox2 MOs alone. Scale bar = 50 μm. |
|
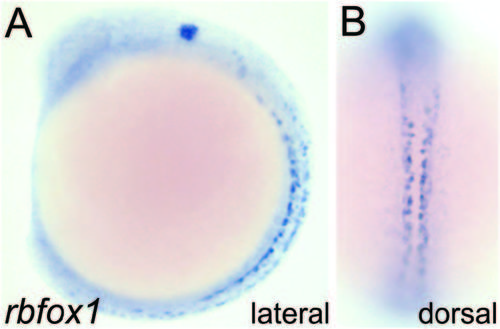
Rbfox1 is expressed in a neural-specific pattern during embryo development. (A, B) rbfox1 transcript is detected in neural cell types by in situ hybridization (shown here at 14 hpf in lateral and dorsal views). Our data is consistent with the work of others showing predominant neural expression through the first 5 days of development (Kurrasch et al., 2007; Thisse, 2004). EXPRESSION / LABELING:
|
|
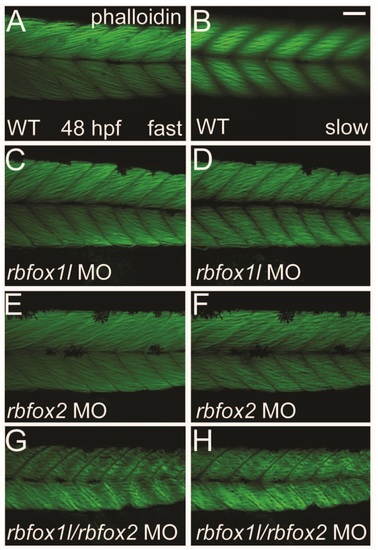
Depletion of Rbfox1l or Rbfox2 alone does not alter the striated appearance of myofibrils. Phalloidin labeling of thin filaments in skeletal muscle of WT embryos reveals normally striated fibers in both fast and slow muscle (A-B). Fiber arrangment of rbfox1l morphants was mildly affected at doses of 9 ng (C-D), whereas rbfox2 morphant fibers had a normal appearance (E-F). In contrast, halved doses of 4.5 ng each of rbfox1l and rbfox2 sbMOs, when co-injected, resulted in highly disorganized, wavy fibers (G-H). Scale bar = 50 µm. PHENOTYPE:
|

Unillustrated author statements PHENOTYPE:
|
Reprinted from Developmental Biology, 359(2), Gallagher, T.L., Arribere, J.A., Geurts, P.A., Exner, C.R., McDonald, K.L., Dill, K.K., Marr, H.L., Adkar, S.S., Garnett, A.T., Amacher, S.L., and Conboy, J.G., Rbfox-regulated alternative splicing is critical for zebrafish cardiac and skeletal muscle functions, 251-61, Copyright (2011) with permission from Elsevier. Full text @ Dev. Biol.