- Title
-
Cardiac conduction is required to preserve cardiac chamber morphology
- Authors
- Chi, N.C., Bussen, M., Brand-Arzamendi, K., Ding, C., Olgin, J.E., Shaw, R.M., Martin, G.R., and Stainier, D.Y.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
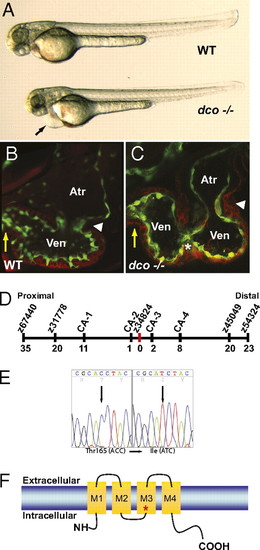
dococs226 Mutants exhibit pericardial edema and cardiac failure due to dysynchronous contraction of the ventricle. (A) Brightfield micrographs of 48 hpf WT (WT) and dcos226 mutant (dco -/-) embryos. Black arrow points to pericardial edema. (B and C) Confocal micrographs of 60 hpf Tg(flk1:EGFP)s843 (B) WT and (C) dcos226 mutant hearts (endothelium green) stained with rhodamine phalloidin (myocardium red). dco Mutant ventricles fail to contract coordinately and exhibit disorganized chamber walls. White arrowheads point to atrioventricular canal; yellow arrows point to outflow tract; white asterisk denotes aberrant ventricular wall contraction. (D) Genetic map of the dco region. Numbers below SSLP markers indicate recombination events out of 922 diploid embryos examined. (E) Sequencing of cx46 cDNA revealed a C-to-T change at base pair 494 in the s226 mutant allele, resulting in a Thr-to-Ile substitution at residue 165. (F) Schematic diagram illustrating modular structure of Cx46. A connexon consists of four transmembrane domains (M1–M4). The M3 domain is the major pore-lining domain. Red asterisk marks site affected by s226 mutation. |
|
Optical mapping/calcium imaging of dococs226 mutants reveals disrupted cardiac conduction. (A–C) Optical maps of calcium excitation during a single cardiac cycle, represented by isochronal lines every 20 ms in 60 hpf embryos carrying the Tg(cmlc2:gCaMP)s878 reporter and (A) WT at the dco locus (WT), (B) dcos226 homozygous mutant (dco), or (C) WT with dcos226 mutant cardiomyocytes carrying the Tg(cmlc2:dsRed)s879 reporter in the ventricle (dco → WT). Black arrow shows normal direction of ventricular cardiac conduction. Numbers indicate temporal sequence of calcium excitation. Color bar chart fluorescence intensity changes on a scale of 0 to 100. Cardiac conduction across the WT ventricle proceeds uniformly from the atrioventricular canal (AV) to the outflow tract (OT), whereas cardiac conduction travels aberrantly through the dcos226 mutant ventricle. Transplanted dcos226 mutant cardiomyocytes disrupt the organized AV to OT conduction in WT ventricle. White asterisk marks location of transplanted dco mutant cardiomyocytes in WT ventricle, as illustrated in D′ and D′′. (D and D′′) Fluorescent micrographs of the dco → WT mosaic heart imaged in C. (D) Green fluorescence shows the WT host ventricle; (D′) donor-derived dcos226 mutant cardiomyocytes expressing Tg(cmlc2:dsRed)s879. (D′′) Overlay of green and red fluorescence images reveals that the donor-derived dcos226 mutant cardiomyocytes are located at the outer curvature of the ventricle near the AV canal, where cardiac conduction is disrupted in the WT host ventricle (C). |
|
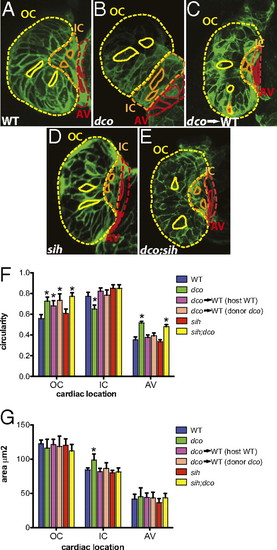
Cardiac conduction, independent of hemodynamic flow or cardiac contraction, is required for cardiomyocyte morphogenesis. (A–E) Confocal images of 60 hpf embryos carrying the Tg(cmlc2:ras-eGFP)s883 reporter and (A) WT at the dco locus (WT), (B) dcos226 homozygous mutant (dco), (C) WT with dcos226 mutant cardiomyocytes carrying the Tg(cmlc2:dsRed)s879 reporter in the ventricle (dco → WT), (D) sih homozygous mutant (sih), or (E) dco;sih double homozygous mutant (dco;sih). Outer curvature (OC), inner curvature (IC), and atrioventricular canal (AV) are outlined in yellow, orange, and red dashed lines, respectively. Representative cardiomyocyte shapes for each area are outlined with solid lines. Bar graphs represent (F) cell morphology/circularity and (G) surface area measurements of Tg(cmlc2:ras-eGFP)s883 cardiomyocytes at 60 hpf from the outer and inner curvatures of the ventricle as well as the AV canal. Bar height indicates mean for a dataset; error bars indicate SE. *Statistically significant differences compared with WT (P < 0.001). Defects in electrical conduction result in aberrant cardiomyocyte morphogenesis. |
|
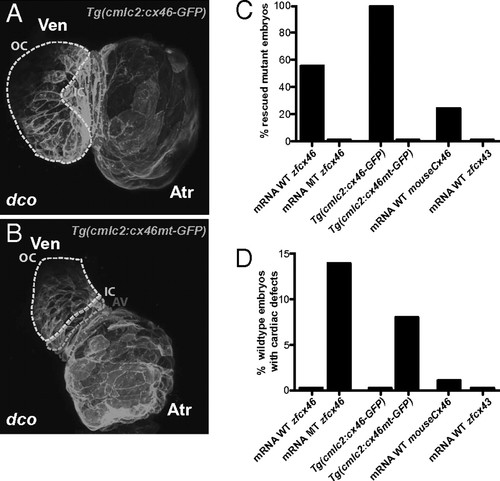
dco Cardiac phenotype is rescued by RNA injection of Cx46 orthologs as well as by myocardial specific expression of zebrafish cx46. (A and B) Myocardial specific expression of WT [Tg(cmlc2:cx46-GFPs882)] and mutant [Tg(cmlc2:cx46mt-GFPs925)] zebrafish Cx46-GFP fusion proteins lead to cell membrane enrichment. Outer curvature (OC), inner curvature (IC), and atrioventricular canal (AV) are outlined by dashed lines. Atr, atrium; Ven, ventricle. (C) Injection of WT zebrafish (WT zfcx46) or mouse Cx46 (mouseCx46) mRNA as well as myocardial specific expression of WT zebrafish Cx46-GFP fusion proteins [Tg(cmlc2:cx46-GFPs882)] rescued the dco cardiac phenotype. On the other hand, injection of zebrafish cx46s226 (MT zfcx46) or cx43 (zfcx43) mRNA as well as myocardial specific expression of zebrafish Cx46s226-GFP [Tg(cmlc2:cx46mt-GFPs925)] failed to rescue the dco cardiac phenotype. (D) However, injection of zebrafish cx46s226 mRNA or myocardial specific expression of zebrafish Cx46s226-GFP [Tg(cmlc2:cx46mt-GFPs925)] in WT embryos resulted in dco cardiac phenotypes. In addition, RNA overexpression of WT murine Cx46 caused a dco cardiac phenotype in a small percentage of WT embryos. |
|
cx46 Expression and morpholino knockdown confirms that cx46 is the affected gene in dco mutants. (A–D) Whole-mount RNA in situ hybridization reveals cx46 expression in the heart and lens. Expression of cx46 at (A) 36, (B) 42, (C) 48, and (D) 72 hpf. A, atrium; V, ventricle. (E) cx46 and β-Actin control RT-PCR from FACS-sorted myocardial and endocardial cells at 36, 48, and 72 hpf. (F) Morpholino knockdown of cx46 recapitulates dco cardiac phenotype. Of WT embryos injected with an ATG cx46 morpholino, 88% are indistinguishable from dco mutant embryos and display severe impairment of cardiac conduction (n = 125/142). EXPRESSION / LABELING:
PHENOTYPE:
|