- Title
-
Bbs8, together with the planar cell polarity protein Vangl2, is required to establish left-right asymmetry in zebrafish
- Authors
- May-Simera, H.L., Kai, M., Hernandez, V., Osborn, D.P., Tada, M., and Beales, P.L.
- Source
- Full text @ Dev. Biol.
|
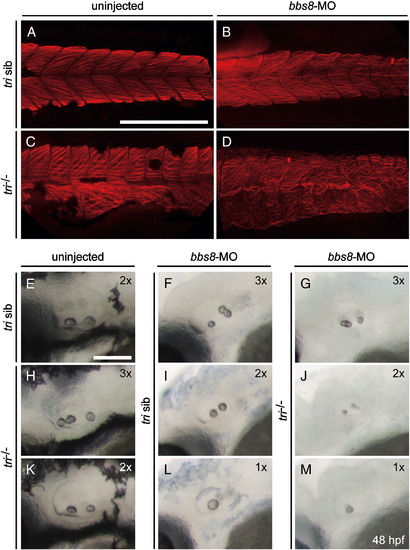
Bbs8 interacts with Vangl2 during zebrafish development. Injection of bbs8-MO at the 1-2-cell stages into trilobite (tri) zebrafish. A-F) At 30 hpf, defective CE cell movement of tri-/- was enhanced after injection of bbs8-MO, resulting in severe body axis compression. Inset C-F) Ventral view of zebrafish embryos at 48 hpf. Note varied spacing between eyes. G-N) Visualisation of somites (myoD) and rhombomeres 3 and 5 (krox20) by in situ hybridisation at the 8-somite stage. Patterning is unaltered in tri siblings. bbs8-MO injected tri-/- embryos had more severe CE defects as evidenced by compressed somites and a wider presumptive neural tube, and a shorter distance between rhombomere 5 and the first somite. Views: lateral (A–G, K, I, M), dorsal (H, L, J, N). PHENOTYPE:
|
|
Bbs8 and Vangl2 act on normal actin organisation and otolith formation. A-D) Visualisation of F-actin in the somite region of zebrafish embryos at 48 hpf by phalloidin. A) Control embryo. B) Injection of bbs8-MO caused undulation of actin filaments. C) In tri-/- embryo the dorsal somites became broad and rectangular with an altered pattern of actin bundles, while the ventral somites were disorganised. D) Injection of bbs8-MO into tri-/- background totally disrupted actin organisation. E-M) Observation of otoliths in zebrafish inner ear at 48 hpf. 1x, 2x, and 3x represent 1, 2 or 3 otoliths respectively. E) Control embryo with two otoliths. H, K) tri-/- embryos showed varied number of otoliths (two or three). F, G, I, J, L, M) Injection of bbs8-MO into tri-/- (G, J, M) or siblings (F, I, L) also resulted in varying numbers of otolith (one to three). Otoliths also appeared to be smaller (G, J, M) than normal. A-D) Lateral view, anterior to the left. A-D) Scale bar: 200 μM; E–M) Scale bar: 100 μM. PHENOTYPE:
|
|
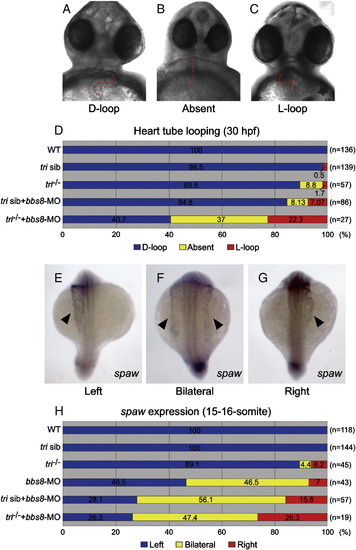
Reduced expression of bbs8 and vangl2/tri results in laterality defects. Cardiac looping and the laterality marker expression. A-D) Cardiac looping at 30 hfp. A) Normal looping (D-loop). B) Reversed looping (L-loop). C) Absent looping. The heart tube is highlighted by a red dotted line (A-C). D) At 30 hpf, injected tri-/- embryos exhibited increased percentages of abnormal cardiac looping. The fractions of embryos of each category are noted in percent along the X-axis. E-G) spaw expression (arrowheads) in 15-16-somite embryos. E) Normal spaw expression on the left lateral mesoderm. F) Bilateral and G) inverted spaw expressions. H) Quantitative representation of spaw expression. In tri embryos injected with bbs8-MO, the expression is randomised. Views: ventral (A-C), dorsal (E-G). EXPRESSION / LABELING:
PHENOTYPE:
|
|
BBS8 and Vangl2 are required for normal Kupffer′s vesicle structure. A) Percentage spaw expression (at 15-16-somite) after bbs8-MO injection targeted to the whole embryo (WE) or the Kupffer′s vesicle (KV). Expression was randomised under both conditions. B-E) Visualisation of cilia (Ac-Tub) in the Kupffer′s vesicle in 8-somite embryos in bbs8-MO injected tri embryos. The number and/or length of the cilia are altered in the experimental conditions. F-G) Table depicting average cilia numbers and cilia length (μm) in injected and uninjected tri embryos. PHENOTYPE:
|
|
Positions of the nucleus and basal body in Kupffer′s vesicle cells. A, B, E, F) Measurements of the nearest nuclei to the apical membrane. In tri-/- embryos, the distance of the nucleus (DAPI) and the apical membrane is increased compared to either bbs8-morphants or sibling controls. C, D) Basal bodies (γ-Tub) and cilia (Ac-Tub) appear to be more detached from the apical membrane of the KV in tri-/- embryos. G) Table depicting nuclear distance from the apical membrane (μm) in injected and uninjected tri embryos. PHENOTYPE:
|
|
Bbs8 and Vangl2 are required for normal fluid flow in the Kupffer′s vesicle. A) Schematic representation of the site of bead injection into the KV for fluid flow analysis. B) Bright field image of the KV with injected beads (red circles). C-F) Tracked bead movements in live bbs8-MO injected tri embryos at 4–5-somite stages. C) A constant anti-clockwise circular movement as seen from the dorsal side was observed in WT embryos. D, E) Bead movement was disrupted in both bbs8-morphants and tri-/- mutants. F) Abrogation of both vangl2/tri and bbs8 abolishes this bead movement completely. PHENOTYPE:
|
|
In vitro binding analysis of BBS8 and Vangl2. A) Co-immunoprecipitation of BBS8-myc with Vangl2-GFP using an anti-GFP antibody for the immunopercipitation and an anti-myc antibody for the Western blot. Empty-GFP, empty-myc and a random GFP construct were used as negative controls. BBS2-GFP, a known binding partner of BBS8 was used as a positive control. Positive co-IP bands are only observed with Vangl2-GFP and BBS8-myc and in the positive control. B) GST pull-down assay of BBS8 and Vangl2. myc-Vangl2 was pulled-down with BBS8-GST and concentrated, as compared to GST-only control. |
|
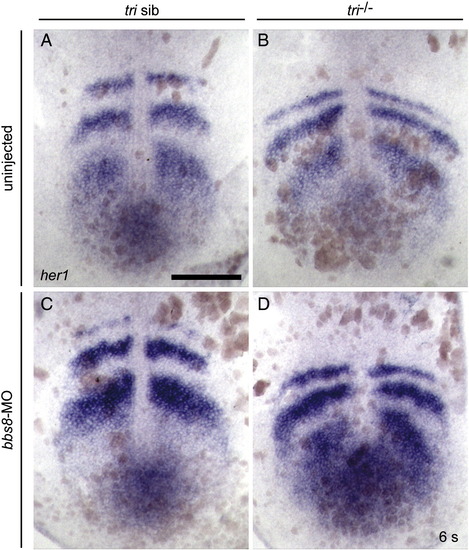
her1 expression in 6ss zebrafish. In situ hybridisation using a probe against her1, did not detect asymmetrical somitogenesis in either the tri-/- or bbs8-MO injected zebrafish. Scale bar: 200 μm. |
|
Cilium/basal body structure in the pronephric duct and otic vesicle is disrupted in tri mutant. Cilia (Ac-Tub, red) and basal bodies (γ-Tub, green) were observed in the pronephric duct (A-D, A′-D′) and otic vesicle (E-H, E′-H′), counterstained with (A-H) or without (A′-H′) nuclei (DAPI, blue). The normally linear organisation of pronephric duct cilia in control siblings (A, A′ insets) is disordered in bbs8-morphants and tri-/- with notable dilation of the pronephric lumen (C, C′ insets and B, B′ insets). These affects are further exacerbated in tri-/- embryos injected with bbs8-MO (D, D′ insets). Similarly, in otic vesicles (E-G insets, E′-G′ insets), the classical oblong shape in control sibling embryos (E, E′ insets) is malformed to a more oval shape in tri-/- (F, F′, insets) and an undersized sphere in bbs8-morphants (G, G′ insets). Again these defects are enhanced in tri-/- embryos injected with bbs8-MO, generating even smaller spherical vesicles with notable compaction of cilia/basal bodies (H, H′ insets). Taken together, these data suggest vangl2/tri and bbs8 are required for normal cilium/basal body structure in a variety of tissues. Scale bar: 200 μm. |
|
Apical specification at the Kupffer′s vesicle is largely unaffected in tri mutant and bb8-morphant. Visualisation of apical membrane and cilia in the Kupffer′s vesicle using anti-ZO-1 and anti-α-acetylated tublin antibodies in the control (A), tri-/- (B), bbs8-morphant (C) and tri-/- injected with bbs8-MO (D) embryos. ZO-1 was observed on the cell membrane lining the KV (A-D), suggesting that apical specification is mostly intact in all conditions. Also note that the near-complete oval outline of the KV in wild-type (A) is partially disrupted in B-D (Yen et al., 2006). |
Reprinted from Developmental Biology, 345(2), May-Simera, H.L., Kai, M., Hernandez, V., Osborn, D.P., Tada, M., and Beales, P.L., Bbs8, together with the planar cell polarity protein Vangl2, is required to establish left-right asymmetry in zebrafish, 215-225, Copyright (2010) with permission from Elsevier. Full text @ Dev. Biol.