- Title
-
In vivo birthdating by BAPTISM reveals that trigeminal sensory neuron diversity depends on early neurogenesis
- Authors
- Caron, S.J., Prober, D., Choy, M., and Schier, A.F.
- Source
- Full text @ Development
|
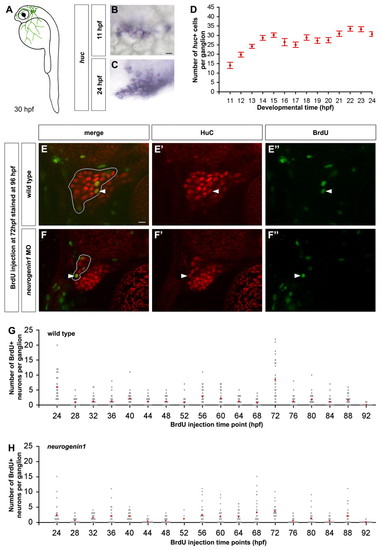
Continuous neurogenesis in the zebrafish trigeminal sensory ganglia. (A) As schematized in a 30 hpf zebrafish embryo, the trigeminal sensory ganglia (green) are located on each side of the head posterior to the eyes. Trigeminal sensory neurons innervate the head and part of the yolk. (B-D) Wild-type embryos were hybridized with a huc antisense probe to count the number of trigeminal sensory neurons per ganglion. The first neurons appear at 11 hpf forming small clusters (B). During subsequent development, the number of neurons per ganglion increases (C). Side view, anterior towards the left; red asterisks label single neurons. Scale bar: 10 μm. The graph represents the average number of neurons per ganglion at different developmental stages ranging from 11 hpf to 24 hpf (D). An individual ganglion contains an average of 14 neurons at 11 hpf and reaches an average size of 30 neurons at 24 hpf. Error bars indicate s.e.m. (n=15). (E-H) To measure the number of neurons added to the trigeminal sensory ganglion after 24 hpf, embryos were injected once with BrdU, fixed at 96 hpf and immunostained for HuC (red) and BrdU (green). Double labeled cells in the trigeminal sensory ganglia of wild-type (E) or neurogenin1 morphant (F) embryos injected with BrdU at 72 hpf are indicated with a white arrowhead. Side view, anterior towards the left; white outline delimits the trigeminal sensory ganglion from the anterior lateral line. Scale bar: 10 μm. The graphs represent the number of neurons per ganglion born after the BrdU injection time point in wild-type (G) and neurogenin1 morphant (H) embryos. Injections were performed at different times ranging from 24 hpf to 92 hpf. Gray dots represent individual samples and red dots represent the average number of BrdU-positive trigeminal sensory neurons found per ganglion at each injection time point (n=30). EXPRESSION / LABELING:
|
|
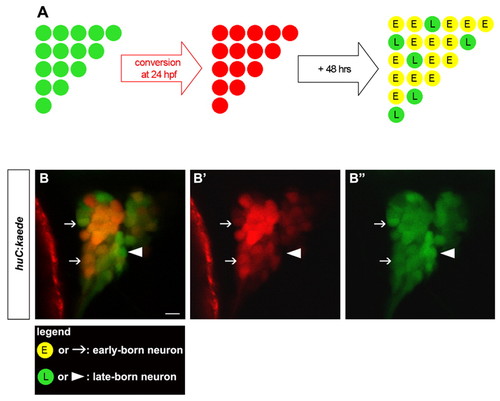
In vivo birthdating analysis of trigeminal sensory neurons using BAPTI. Embryos carrying the huc:kaede transgene were analyzed using the birthdating analysis by photoconverted fluorescent protein tracing in vivo method (BAPTI). (A) As schematized, the trigeminal sensory neurons initially appear green. Following illumination of 24 hpf embryos with ultraviolet light, huc:kaedegreen is converted to huc:kaedered and all neurons born prior to 24 hpf appear red. After 48 hours incubation, neurons born before 24 hpf retain huc:kaedered and express de novo huc:kaedegreen, whereas neurons born after 24 hpf express only huc:kaedegreen. Early-born neurons (E) appear red and green (yellow); late-born (L) neurons appear green only. (B) Converted embryos were imaged at 72 hpf. Early-born neurons are identifiable by their red and green signals (arrow), whereas late-born neurons are identifiable by their green only signal (arrowhead). Neurons with weak (top arrow) or strong red signals (bottom arrow) were counted as early-born neurons. The entire trigeminal sensory ganglion was imaged by confocal microscopy. At this plane of confocal section only one late-born neuron is visible (arrowhead). Side view, anterior towards the left. Scale bar: 10 μm. EXPRESSION / LABELING:
|
|
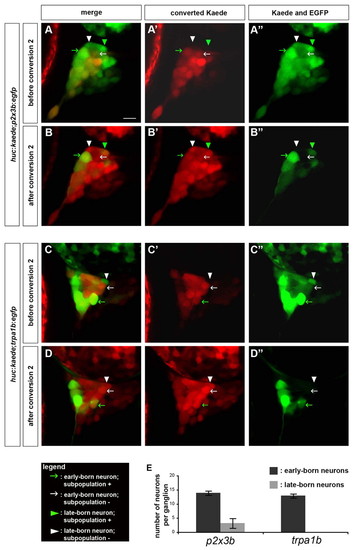
Late-born but not early-born neurons are restricted in their fate. Embryos carrying the huc:kaede transgene, together with either the p2x3b:egfp or the trpa1b:egfp transgene were analyzed using BAPTISM. (A-D) huc:kaede was first converted at 24 hpf, imaged at 72 hpf (A,C) and converted a second time (B,D). (A,B) In huc:kaede;p2x3b:egfp embryos, early-born neurons either express the subpopulation marker (green arrow) or do not (white arrow), and late-born neurons either express the subpopulation marker (green arrowhead) or do not (white arrowhead). (C,D) In huc:kaede;trpa1b:egfp embryos, early-born neurons either express the subpopulation marker (green arrow) or do not (white arrow). Late-born neurons do not express the subpopulation marker (white arrowhead). Side view, anterior towards the left. Scale bar: 10 μm. (E) The histogram represents the number of neurons per trigeminal sensory ganglion expressing the p2x3b or trpa1b subpopulation markers derived from either early-born neurons (dark gray) or late-born neurons (light gray). The error bars refer to the s.e.m. (n=7). EXPRESSION / LABELING:
|
|
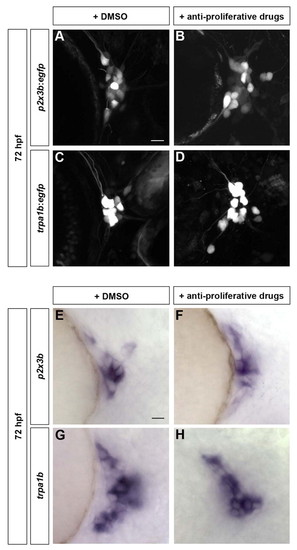
Early-born trigeminal sensory neurons contribute to various subpopulations independently of late-born neurons. Subpopulation markers were analyzed in embryos lacking late-born trigeminal sensory neurons. (A-H) Embryos were incubated from 24 hpf to 72 hpf with 2% DMSO alone (A,C,E,G) or 20 mM hydroxyurea and 150 μM aphidicolin (B,D,F,H). Embryos carrying the p2x3b:egfp (A,B) or trpa1b:egfp (C,D) transgene were imaged at 72 hpf. Wild-type embryos were fixed at 72 hpf and in situ hybridization was performed for p2x3b (E,F) and trpa1b (G,H). Side view, anterior towards the left. Scale bars: 10 μm. EXPRESSION / LABELING:
|
|
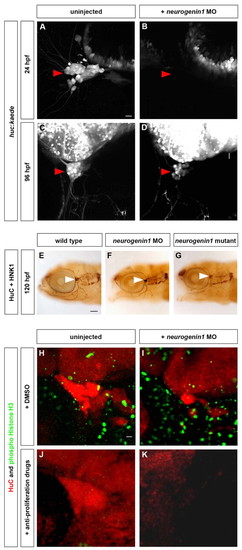
The trigeminal sensory ganglia of neurogenin1 mutant and morphant embryos are solely formed from late-born neurons. Neurogenin1-depleted embryos develop smaller trigeminal sensory ganglia formed from late-born neurons only. (A-D) Embryos carrying the huc:kaede transgene were injected with 6 ng of neurogenin1 antisense morpholino (B,D) or uninjected (A,C). At 24 hpf, the trigeminal sensory ganglia are visible in uninjected embryos by the expression of Kaede (A,C) but no trigeminal sensory neurons are detectable in the neurogenin1 morphants at 24 hpf (B). At 96 hpf, the trigeminal sensory ganglia are visible in neurogenin1 morpholino-injected embryos (D) but contain fewer neurons than uninjected embryos (C). Side view, anterior towards the left. Scale bar: 10 μm. (E-G) The morphology of the neurons of the trigeminal sensory ganglia was analyzed by immunostaining in wild-type (E), neurogenin1 morphant (F) and neurogenin1 mutant (G) embryos with HuC, a pan-neuronal marker, and HNK-1, a marker labeling the cell surface of sensory neurons. White arrowheads indicate the trigeminal sensory ganglia. Side view, anterior towards the left. Scale bar: 100 μm. (H-K) To determine whether the trigeminal sensory ganglia in neurogenin1 morphant embryos are partly formed from early-born neurons, embryos were treated with 2% DMSO alone (H,J) or with 20 mM hydroxyurea and 150 μM aphidicolin (I,K) at 24 hpf. HuC staining (red) labels the trigeminal sensory ganglia. Staining for the mitotic marker phospho-histone H3 (green) was used to monitor the number of proliferating cells in the whole embryos. Proliferation was not affected in mock-treated embryos (H,J) but was significantly reduced in treated embryos (I,K). No trigeminal sensory neurons are detectable in the neurogenin1 morphant embryos treated with the anti-proliferative drugs (K), in contrast to the mock-treated neurogenin1 morphant (I) or wild-type embryos (H,J). Side view, anterior towards the left. Scale bar: 10 μm. |
|
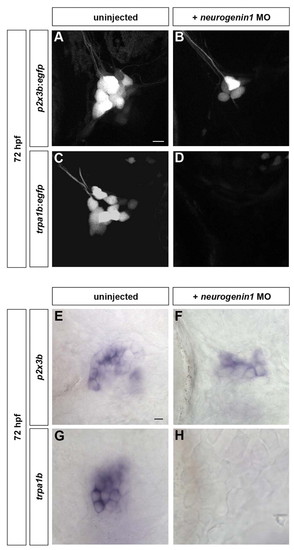
Cell fate restriction of late-born trigeminal sensory neurons is independent of early-born neurons. Subpopulation markers were analyzed in embryos lacking early-born trigeminal sensory neurons. (A-D) Embryos carrying the p2x3b:egfp (A,B) or trpa1b:egfp (C,D) transgene were injected with 6 ng of neurogenin1 morpholino (B,D) and imaged at 72 hpf. Although both p2x3b-expressing and trpa1b-expressing neurons are visible in uninjected larvae (A,C), only p2x3b-expressing are detectable in neurogenin1 morphant larvae (B). No trpa1b-expressing neurons were detected in these larvae (D). (E-H) Wild-type (E,G) and neurogenin1 morphant (F,H) larvae were stained by in situ hybridization at 96 hpf for p2x3b (E,F) or trpa1b (G,H). No trpa1b-expressing neurons were detected in neurogenin1 morphant larvae (H). Side view, anterior towards the left. Scale bars: 10 μm. EXPRESSION / LABELING:
|
|
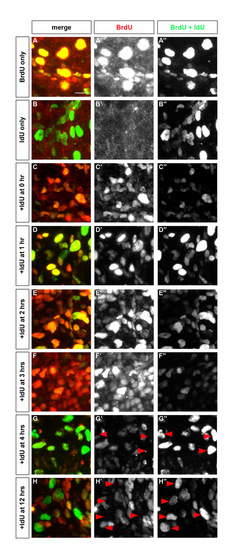
BrdU remains available for 4 hours after injection. To measure the perdurance of BrdU in zebrafish embryos, BrdU was chased with IdU. Embryos were injected with BrdU and/or IdU and were fixed 2 hours after the last injection. Embryos were stained with an antibody that recognized BrdU only (AbD Serotec) and an antibody that recognized both BrdU and IdU (Becton Dickinson). Primary antibodies were detected using an anti-rabbit antibody coupled with rhodamine and an anti-mouse antibody coupled with FITC (Jackson ImmunoLab), respectively. Therefore, cells labeled with BrdU only or with BrdU and IdU have a red and green signal (yellow in merged image), whereas cells that are labeled with IdU only have a green signal. (A-C) Staining of embryos injected with BrdU only (A), IdU only (B), or both (C), revealed that the anti-BrdU antibody specifically recognized BrdU, whereas the anti-BrdU/IdU antibody recognized both nucleotides. (D-H) Embryos were injected first with BrdU at 24 hpf and injected again with IdU 1 hour (D), 2 hours (E), 3 hours (F), 4 hours (G) and 12 hours (H) following the BrdU injection. When IdU was injected 4 hours after the BrdU injection, several green stained cells were visible throughout the embryo, indicating that BrdU is no longer available to be incorporated 4 hours after injection. Side view, anterior towards the left. |
|
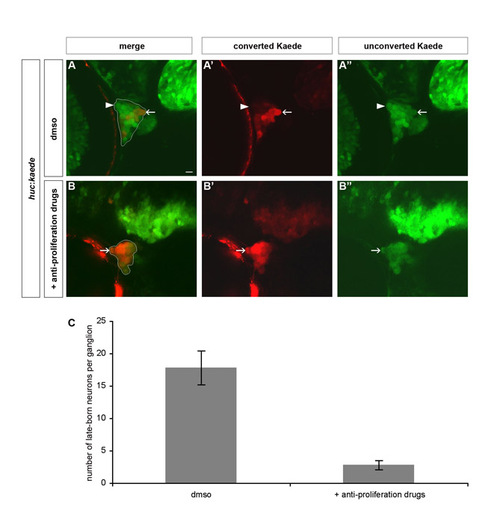
Blocking proliferation eliminates late-born neurons in trigeminal sensory ganglia. To remove late-born neurons from the trigeminal sensory ganglia, proliferation was blocked after 24 hpf by treating embryos with anti-proliferative drugs. (A,B) Wild-type embryos were treated with 2% DMSO alone (A) or 20 mM hydroxyurea and 150 μM aphidicolin (B). Embryos were analyzed using BAPTI at 72 hpf. Late-born neurons contain only green, unconverted, Kaede (white arrowhead), whereas early-born neurons also contain red, converted, Kaede (white arrow). (C) The chart represents the number of late-born neurons per trigeminal sensory ganglion in treated and mock-treated embryos. Late-born neurons are severely reduced or absent upon treatment with the anti-proliferative drugs. The error bar refers to the standard error. EXPRESSION / LABELING:
|
|
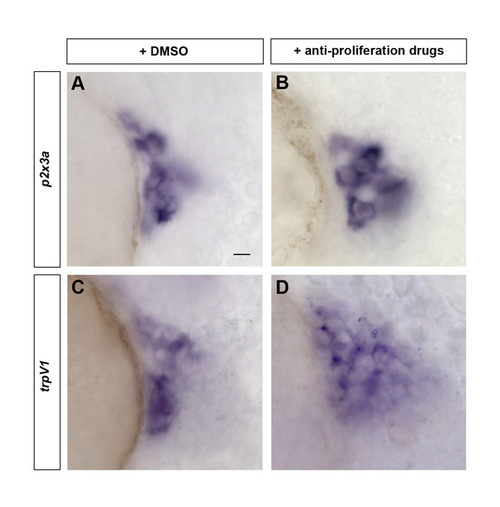
Early-born trigeminal sensory neurons contribute to various subpopulations independently of late-born neurons. Subpopulation markers were analyzed in embryos lacking late-born trigeminal sensory neurons. (A-D) Wild-type embryos were incubated from 24 hpf to 72 hpf with 2% DMSO alone (A,C) or 20 mM hydroxyurea and 150 μM aphidicolin (B,D). Treated embryos were fixed at 72 hpf and stained by in situ hybridization for p2x3a (A,B) and trpv1 (C,D). Side view, anterior towards the left. Scale bar: 10 μm. EXPRESSION / LABELING:
|
|
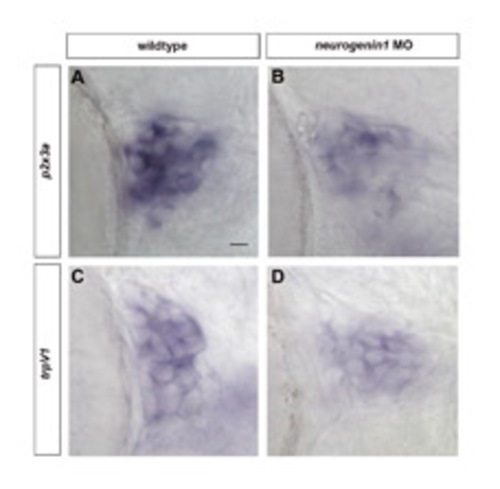
Cell fate restriction of late-born trigeminal sensory neurons is independent of early-born neurons. Subpopulation markers were analyzed in embryos lacking early-born trigeminal sensory neurons. (A-D) Wild-type (A,C) and neurogenin1 morphant (B,D) embryos were fixed at 72 hpf and stained by in situ hybridization for p2x3a (A,B) or trpv1 (C,D). Side view, anterior towards the left. Scale bar: 10 μm. EXPRESSION / LABELING:
|
|
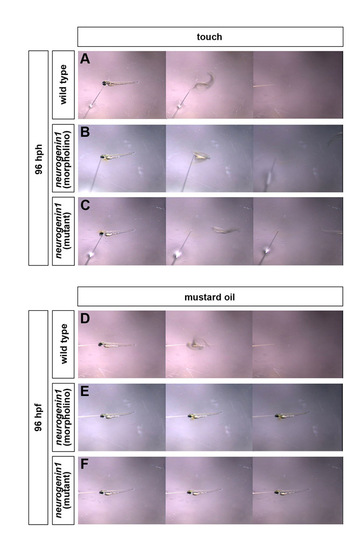
Birthdate affects the sensory modalities of the trigeminal sensory ganglia. (A-F) Wild-type (A,D), neurogenin1 morphant (B,E), and neurogenin1 mutant (C,F) larvae were tested for their response to touch (A-C) and allyl isothiocyanate (D-F). Fish larvae were recorded using a video camera mounted on a dissection scope. Larvae were considered responsive if they instantaneously escaped the source of the stimulus. Touch stimulus was delivered by touching the head of the fish with a glass needle. Wild-type and neurogenin1-depleted 96 hpf larvae are responsive to touch (A-C). Response to allyl isothiocyanate diluted in DMSO (1:100) was tested. Wild-type (D) but neither neurogenin1 morphant (E) nor mutant larvae (F) are responsive to allyl isothiocyanate. For quantification, see Table 1. |