- Title
-
Development and growth of organs in living whole embryo and larval grafts in zebrafish
- Authors
- Kawasaki, T., Maeno, A., Shiroishi, T., Sakai, N.
- Source
- Full text @ Sci. Rep.
|
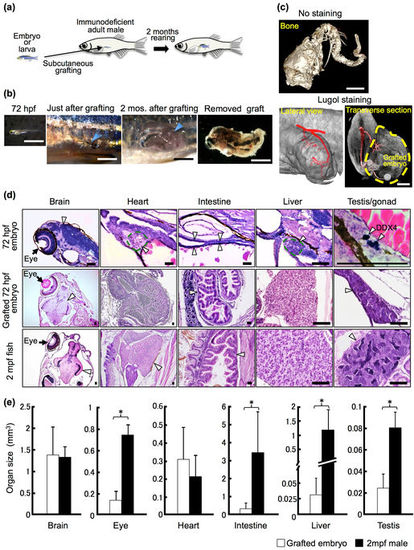
Development of grafted 72 hpf embryos and organ growth in rag1 mutant hosts. (a) Schematic of whole-embryo transplantation. (b) Representative 72 hpf embryos and grafted embryos. Arrowheads indicate the site of the subcutaneous embryo graft. Scale bar: 2 mm. (c) µ-CT imaging of the grafted embryo 2 months after transplantation. Cranial bones and vertebrae were observed in the grafted embryos without staining. Using Lugol staining, a vascular structure was observed. Note that the posterior cardinal vein (red) of the recipient is connected to the grafted embryo (yellow dotted line). Scale bar: 1 mm. (d) Histology of the 72 hpf embryo, the 72 hpf embryo graft 2 months after transplantation, and the 2 mpf fish. Germ cells of 72 hpf embryos were stained with anti-DDX4 (Vasa) antibody to identify the primitive gonad. Arrowheads indicate each organ as labelled above the panels. Scale bar: 80 µm. (e) Comparison of organ size between grafted 72 hpf embryos 2 months after transplantation and normal 2 mpf males. The volume of each organ was calculated from serial sections of grafts (n = 12 brains, 11 eyes, 11 hearts, 12 intestines, 8 livers, and 6 testes) and of 2 mpf males (n = 3). Error bars indicate standard deviation. *p < 0.01 (Student’s t-test). |
|
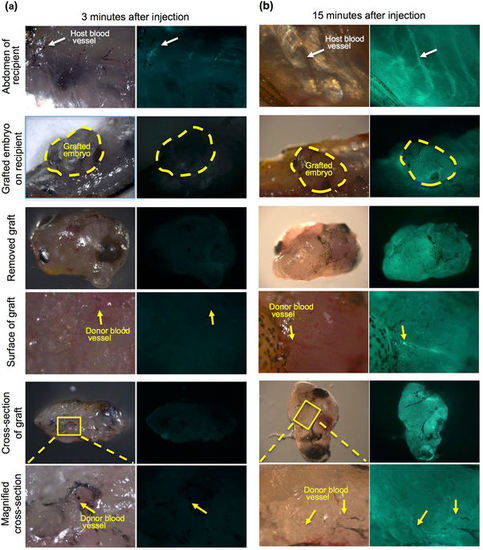
Microinjection of FITC-dextran into the recipient heart. To examine vascularization between recipients and grafted embryos, FITC-conjugated dextran solution was injected into the hearts of living recipients in which 72 hpf embryos had been transplanted 3 months previously. (a) At 3 minutes after injection, slight fluorescence of the recipient blood vessels (white arrow) was observed in the recipient abdomen but not in grafted embryos (yellow dotted line). No fluorescence in the blood vessels was observed in the grafted embryo (yellow arrows). The recipient heart in which FITC-dextran was injected is outside the frame of this panel. Most of the FITC-dextran has not reached the abdomen at 3 minutes after injection. (b) At 15 minutes after injection, strong fluorescence was observed in the recipient blood vessels (white arrow) of the abdomen and the grafted embryos (yellow dotted line). This strong fluorescence was also observed in the blood vessels of the grafted embryo (yellow arrows). These results suggest that recipient blood vessels are connected with grafted embryos. |
|
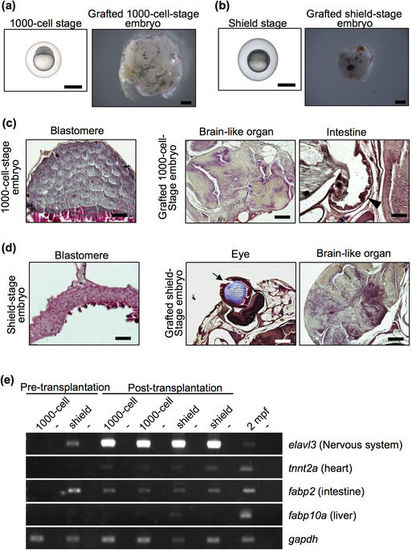
Failure of organ formation in grafted blastular and gastrular embryos. (a,b) Representative 1000-cell-stage (3 hpf) embryos (a), shield-stage (6 hpf) embryos (b) and grafted embryos two months after transplantation. Both embryo stages were subcutaneously transplanted into rag1 mutants. Scale bar: 1 mm. (c,d) Histology of 1000-cell-stage embryos (c) and shield-stage embryos (d) before and after transplantation. Sections were stained with haematoxylin and eosin. Although brain-like organs with an irregular shape and cell body arrangement were observed in all of the grafted early-stage embryos, other organs were not observed, except an eye (arrow) and intestine (arrow head) in a few grafted embryos. Scale bars: 200 µm (brain-like organ and eye), 100 µm (intestine), 50 µm (blastula and gastrula). (e) RT-PCR analysis of organ-specific genes in the grafted blastular and gastrular embryos at 2 months post-transplantation. Each experiment was conducted using two independent samples. Wild-type zebrafish at 2 mpf were used as positive controls. Negative controls lacking reverse transcriptase are located in alternating lanes (−). gapdh was used as a positive control. |
|
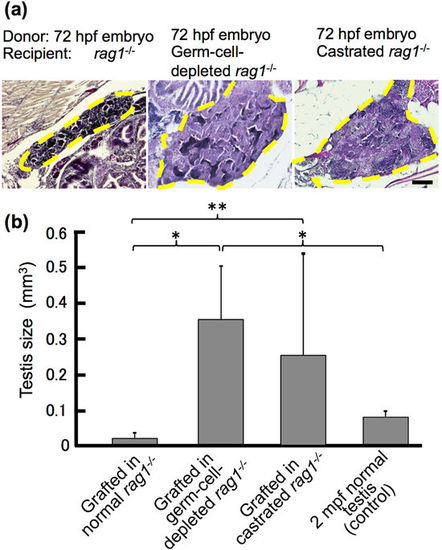
Growth of testis in 72 hpf embryos grafted into germ cell-depleted and castrated rag1 mutants after 2 months. (a) Histology of testes of 72 hpf embryos grafted into normal, germ cell-depleted, and castrated rag1 mutants. Dotted lines represent testes of the grafts. Scale bar, 100 µm. (b) Comparison of testis size between 2 mpf normal testes and grafts into normal, germ cell-depleted and castrated rag1 mutants. Testes volume was calculated from graft serial sections (n = 9 normal, n = 7 germ cell-depleted and n = 5 castrated) and of 2 mpf males (n = 3). Error bars indicate the standard error. *p < 0.01; **P < 0.05 (paired t-test). |
|
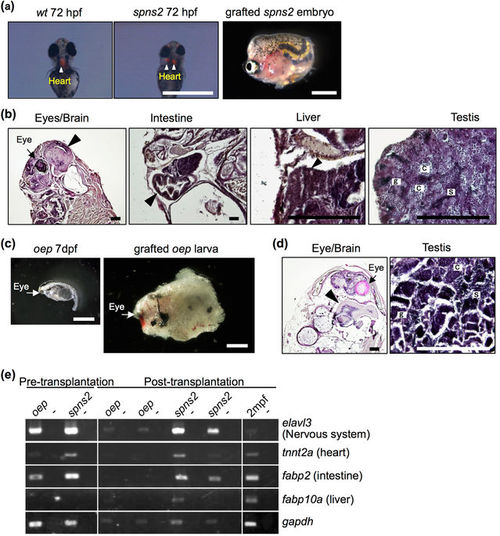
Development of lethal mutant embryos and larvae grafted into rag1 mutants. (a,c) Morphology of 72 hpf spns2 mutant embryos (a) and 7 dpf oep mutant larvae (c) before and after transplantation into germ cell-depleted rag1 mutants. The spns2 mutant has cardia bifida (two hearts) as determined by cmlc2-driven mRFP expression (arrowheads), and the oep mutant exhibits a single eye (arrows). Scale bar: 1 mm. (b,d) Histology of organs found in grafted spns2 mutant embryos (b) and oep mutant larvae (d) at 2 months after transplantation. Arrowheads indicate each organ as labelled above the panels. Note that testes of the grafted spns2 and oep mutants contained all stages of spermatogenic cells: spermatogonia (g), spermatocytes (c), and sperm (s). The heart was not observed in grafted spns2 mutant embryos (b). Scale bar: 200 µm. (e) RT-PCR analysis of grafted spns2 and oep mutants at 2 months post-transplantation. Wild-type zebrafish at 2 mpf were used as a positive control. Negative controls lacking reverse transcriptase are in alternating lanes (−). Each experiment was conducted using two independent samples. |
|
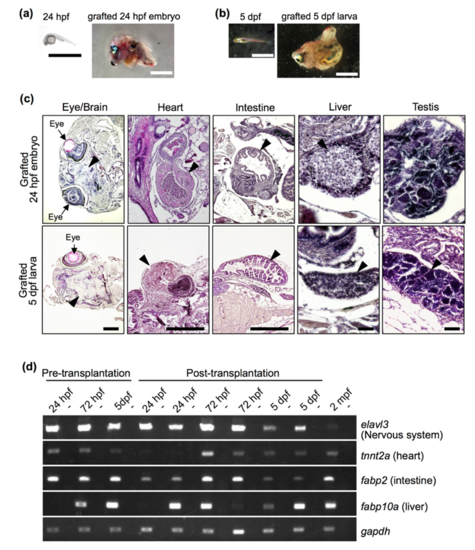
Development of grafted 24 hpf embryos and 5 dpf larvae at 2 months post-transplantation. (A, B) Morphology of 24 hpf embryos (A) and 5 dpf larvae (B) before and after transplantation. Scale bar: 2 mm. (C) Histology of grafted 24 hpf embryos and 5 dpf larvae. Sections were processed with haematoxylin and eosin staining, and representative major organs are shown. Note that morphologically distinct organs developed from 24 hpf embryos and 5 dpf larvae. Arrowheads indicate each organ, as labelled above the panels. Photos of the heart, liver and testis were from grafts in which those organs developed. Scale bars: 500 µm (brain, heart and intestine) and 50 µm (liver and testis). (D) RT-PCR analysis of organ-specific genes in grafted 24 hpf and 72 hpf embryos and 5 dpf larvae at 2 months post-transplantation. Duplicate experiments were performed with independent samples. Wild-type zebrafish at 2 mpf was used as a positive control. Negative controls lacking reverse transcriptase are shown in alternating lanes (-). gapdh was used as a positive control. |