- Title
-
A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides
- Authors
- Voelz, K., Gratacap, R.L., Wheeler, R.T.
- Source
- Full text @ Dis. Model. Mech.
|
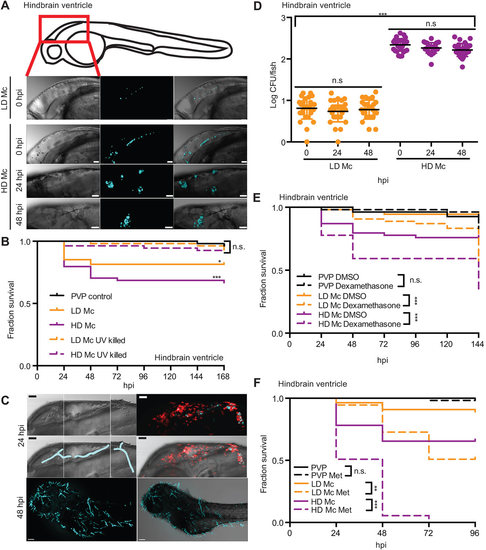
Zebrafish larvae are susceptible to the mucormycete M. circinelloides NRRL3631 in a hindbrain infection model. (A) AB wild-type zebrafish larva were injected in the hindbrain ventricle at prim-25 stage with a low dose (LD Mc, approximately 10 spores) or a high dose of Mucor circinelloides NRRL3631 (HD Mc, approximately 100 spores) and monitored over time (representative z-stacks: 28, 27, 23 and 40 sections every 3Ám, respectively; scale bar: 40Ám). (B) Injection of LD and HD Mc mucormycete spores into the hindbrain ventricle of prim-25 larvae induced significant mortality. Hindbrain ventricle infection with UV-killed spores did not cause significant mortality in AB wild-type larvae compared with the PVP control. (C) Hyphal growth invading the forebrain and ventral muscular layers from posterior hindbrain (representative images of HD Mc infection; top and middle right: DIC montage with filamentous growth highlighted in blue; fluorescent z-stacks: 61 sections every 4.4Ám; scale bar: 50Ám, upper two panels). Dead fish, previously injected with viable spores, presented with filamentous fungal growth (representative image from LD Mc hindbrain injection; cyan, spores/hyphae; red, macrophages; scale bar: 100Ám). (D) Spores remained viable within the zebrafish larvae over the time course of 48h post-infection (h.p.i.). (E,F) Larval immunosuppression increases susceptibility to infections with M. circinelloides NRRL3631. (E) AB wild-type zebrafish larvae treated with dexamethasone after hindbrain ventricle injection showed significantly increased mortality at the low as well as the high infection dose compared with DMSO-treated infected larvae. (F) In comparison to untreated infected Tg(mpeg1:G/U:NfsB-mCherry) larvae, larval treatment with metronidazole (Met) significantly increased susceptibility to low- and high-dose hindbrain ventricle infection with fungal spores. |
|
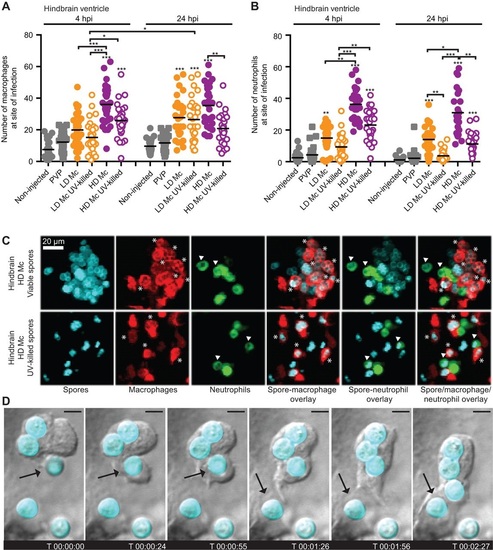
Phagocytes are recruited to the site of infection after spore injection into the hindbrain and interact with spores in vivo. Macrophage (A) and neutrophil recruitment (B) to the site of spore injection in the hindbrain ventricle of Tg(mpeg1:G/U:NfsB-mCherry) and Tg(mpx:GFP) zebrafish larvae, respectively, was observed at 4 and 24h.p.i. Pooled data presented were obtained from three independent experimental repeats with 10 larvae each. (C) Spores can be observed inside macrophages (indicated by asterisks) and neutrophils (indicated by arrowheads) after injection of viable and non-viable spores in the hindbrain ventricle of Tg(mpeg1:G/U:NfsB-mCherry/mpx:GFP) larvae (representative images at 10h 45min and 1h 5min, respectively; z-stack: 15 sections every 7.3Ám; scale bar: 20Ám). (D) Several subsequent events of spore phagocytosis were also observed in an undefined phagocyte in vivo (arrow indicates next spore to be phagocytosed; time is expressed as h:min:s; scale bar: 4Ám; see also Movie 1). |
|
Phagocytes accumulate at the site of live spore injection in the hindbrain infection model. (A) Macrophages and neutrophils accumulate around viable spores (z-stack: 15 sections every 7.3Ám; scale bar: 40Ám; see also Movie 2). (B) Significantly less macrophage and neutrophil clustering was observed in larvae injected with UV-killed spores (z-stack: 15 sections every 7.3Ám; scale bar: 40Ám; see also Movie 3). (C) Formation of clusters around viable spores was correlated with low levels of spore dissemination, whereas non-viable spores did disseminate efficiently throughout the fish (z-stacks: 30 sections every 10.35 and 9.25Ám, respectively, scale bars: 50Ám; zoomed in section: z-stack 31 sections every 1Ám). Time is expressed as h:min:s starting at 1h after injection. |
|
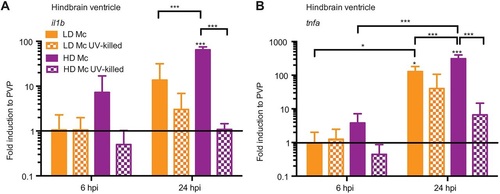
Mucormycete spores induce differential pro-inflammatory cytokine expression dependent on spore viability in the hindbrain infection model. Initial mRNA levels for both cytokines were low at 6h.p.i. (A) but increased after 24h.p.i. (B) for infections with live spores. At 24h.p.i., high-dose infection with viable spores induced significantly higher mRNA levels than infection with a low dose of viable spores and non-viable spores. Infection with non-viable spores did not induce significant cytokine expression at either time point. |
|
A zebrafish larval swim bladder infection model for mucormycosis. (A) AB wild-type zebrafish larvae were injected in the swim bladder 4days post-fertilization (d.p.f.) with a LD or a HD of M. circinelloides NRRL3631. (B) No significant mortality was observed after mucormycete spore injection into the swim bladder 4d.p.f. (PVP versus LD Mc P=0.172; PVP versus HD Mc P=0.403; LD Mc versus HD Mc P=0.561; n=68). (C) Spores remained viable within the zebrafish larvae over the time course of 48h.p.i. (D,E) Macrophages and neutrophils are recruited to the site of infection after injection of viable spores into the swim bladder. Injection of UV-killed spores did not induce significant phagocyte recruitment. (F,G) No significant induction of the pro-inflammatory cytokines il1b or tnfα was detected at 6 or 24h.p.i. with the exception of weak tnfα induction at 24h.p.i. |
|
M. circinelloides virulence can be assessed in immunocompetent zebrafish larvae. (A) Large-spore isolate M. circinelloides CBS277.49 in comparison to the small-spore isolate M. circinelloides NRRL3631. CW, Calcofluor White; scale bars: 10Ám. (B) CBS277.49 at a high but not a low infection dose is significantly more virulent compared with NRRL3631 in AB wild-type zebrafish larvae when injected into the hindbrain ventricle. (C) CBS277.49 at a high but not a low infection dose is significantly more virulent compared with NRRL3631 in AB wild-type zebrafish larvae when injected into the swim bladder. |
|
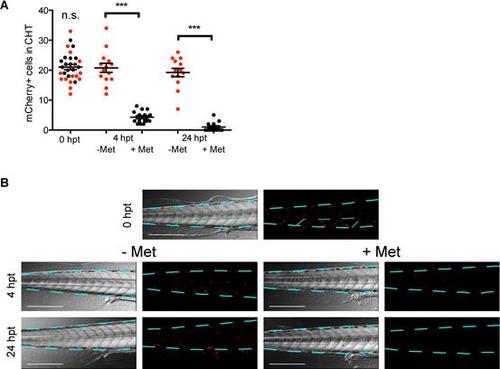
Metronidazole treatment ablates mCherry positive cells in mpeg1-Gal4 x UAS:nfsBmCherry line. To assess the efficacy of macrophage depletion Tg (mpeg1:G/U:NfsB-mCherry) larvae were assessed for the number of mCherry positive cells in the caudal hematopoietic tissue (CHT), 6 somites posterior to the anal vent (green outline in B) before 0 hours post treatment (0 hpt) and after 4 and 24 hours with (+Met, black dots, n=15) or without (-Met, red dots, n=15) Metronidazole. (A&B) There was no significant difference between +Met and ?Met treatment group at 0 hpt and significantly reduced numbers of mCherry positive cells after 4 and 24 hpt in +Met larvae compared to the ?Met larvae (p<0.001). Images are overlays of a single slice in DIC channel and maximum projection (25 slices) in the red channel or red channel only. Scale bar represents 250 Ám and blue dash line outline the body of the fish. |