Fig. 6
- ID
- ZDB-FIG-250311-150
- Publication
- Duque et al., 2024 - Ketamine induces plasticity in a norepinephrine-astroglial circuit to promote behavioral perseverance
- Other Figures
- All Figure Page
- Back to All Figure Page
|
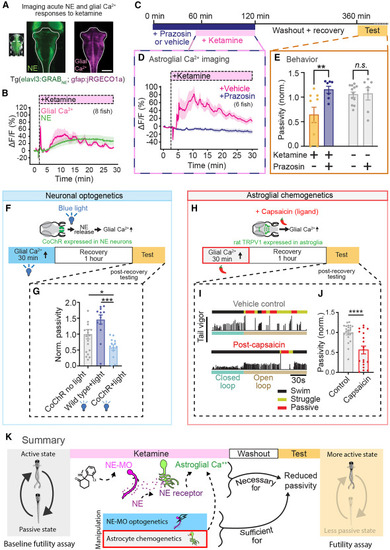
NE-dependent astroglial calcium elevation is required and sufficient for ketamine’s behavioral effects (A) Fluorescence image of a larval zebrafish expressing GRABNE2h under the elavl3 promoter (green) and jRGECO1a (glial Ca2+) under the gfap promoter (pink). Scale bar, 100 μm. (B) Simultaneously imaged intracellular glial Ca2+ dynamics (jRGECO1a signal) and extracellular NE dynamics (GRABNE2h signal). Traces represent mean of fish (n = 8), and shaded regions denote SEM. Shaded region indicates ketamine (200 μg/mL) in bath. (C) Schematic of experiments testing whether ketamine’s effects on astroglial Ca2+ and futility-induced passivity behavior depend on NE signaling. (D) Astroglial Ca2+ (jRGECO1a signal) response to ketamine (200 ug/mL) application in fish treated with 50 μM prazosin (n = 6) or vehicle control (n = 5). (E) Open-loop passivity for fish treated with ketamine, with or without α1 adrenergic blocker prazosin (100 μM). All error bars denote SEM. ∗ p < 0.05, ∗∗ p < 0.01, n.s. p > 0.4. ΔF (Z scored) for each fish was calculated as the difference between mean total fluorescence before treatment subtracted to total fluorescence at a specific time point, divided by the standard deviation of total fluorescence before treatment, for hindbrain mask (D). Two-way ANOVA with Sidak’s multiple comparison test. n = 7 (control-prazosin and ketamine-prazosin), 9 (ketamine-vehicle), 11 (control-vehicle). p = Interaction (0.0140), 0.0057 (ket-prazosin vs. ket-vehicle), 0.9947 (control-vehicle. vs. control-prazosin). (F) Tg(dbh:KalTA4; UAS:CoChR-eGFP) fish expressing the channelrhodopsin CoChR in norepinephrinergic (NE) neurons were continually stimulated optogenetically with blue (488 nm) light for 30 min. Fish were allowed to recover for an hour then assayed for futile swimming. (G) Passivity normalized to clutch, non-stimulated controls. Optogenetic stimulation suppressed futility-induced passivity following recovery compared with non-stimulated clutch controls and non-expression controls stimulated with blue light. One-way ANOVA with Sidak’s multiple comparison test. n = 19 (dbh:CoChr, no light), 14 (wild type, blue light), 13 (dbh:CoChr, blue). p = 0.0004 (WT-Blue light vs. DBH-Blue light), p = 0.0392 (WT-Blue light vs. DBH-no light), p = 0.0392 (DBH-no light vs. DBH-Blue light). (H) Transgenic fish expressing rat TRPV1 in astroglia were treated with capsaicin for 30 min. After 30 min, capsaicin was washed out and fish allowed to recover for 1 h before being tested. (I) Example swimming of capsaicin-treated fish (bottom) and untreated clutch controls (top). Black, yellow, and red segments above swim vigor trace denote regular swimming, struggling, and passivity, respectively. (J) Passivity normalized to untreated clutch controls. Capsaicin-treated fish exhibit decreased futility-induced passivity, although the distribution of passivity duration is bimodal. Mann-Whitney test. n = 22 (control), 19 (capsaicin), p = 0.0005. (K) Summary of findings. |