Fig. s3
|
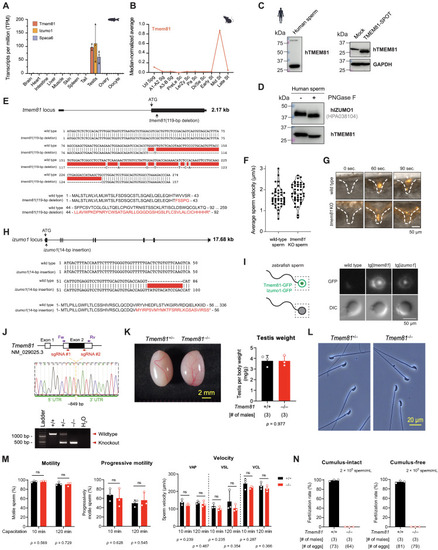
Characterization of expression and function of Tmem81 and Izumo1 in fish and Tmem81 in mice, related to Figure 2 (A) Expression of zebrafish tmem81, izumo1, and spaca6 mRNA in adult tissues (RNA sequencing [RNA-seq] data from Noda et al.15 and Herberg et l.31). (B) Median-normalized level of Tmem81 mRNA expression during mouse spermatogenesis. Tmem81 is expressed in mid-round spermatids. (C) TMEM81 is expressed in human sperm. Left: western blot analysis of human sperm probed for human TMEM81 protein. Right: the human TMEM81 antibody is specific based on western blot analysis of HEK293 cells, which were either mock transfected (negative control; lane 1) or transfected with SPOT-tagged human TMEM81 (lane 2); glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is shown as loading control. (D) Human TMEM81 protein is glycosylated in human sperm. Western blot analysis of human sperm lysate treated with or without peptide-N-glycosidase (PNGase) F to remove protein glycosylation. (E) Zebrafish tmem81 mutant. Top: scheme of the zebrafish tmem81 locus (ENSDART00000062375.5), indicating the start codon (ATG) and the location of the 119-bp deletion present in the tmem81 mutant generated in this study. Exons are depicted as boxes, introns as lines. Middle: partial cDNA sequence alignment of wild-type and mutant tmem81. The tmem81 mutant has a net deletion of 119 bp highlighted in red. Bottom: partial amino acid sequence alignment for wild-type (amino acids 1–92) and mutant Tmem81. Deletion in mutant tmem81 leads to a frameshift that results in a premature stop codon. (F) Average sperm motility for wild-type and tmem81 mutant sperm. Violin plot indicates quartiles. (G) Time-lapse images of sperm approaching the micropyle of wild-type eggs. Representative time series of MitoTracker-labeled wild-type (top) and tmem81 KO (bottom) sperm (orange) approaching the micropyle (white dashed lines). (H) Zebrafish izumo1 mutant. Top: scheme of the zebrafish izumo1 locus (ENSDART00000184278.1), indicating the start codon (ATG) and the location of the 14-bp insertion present in the izumo1 mutant generated in this study. Exons are depicted as boxes, introns as lines. Middle: partial cDNA sequence alignment of wild-type and mutant izumo1. The izumo1 mutant has a 14-bp insertion highlighted in red. Bottom: partial amino acid sequence alignment for wild-type (amino acids 1–56) and mutant Izumo1. Insertion in mutant izumo1 leads to a frameshift that results in a premature stop codon. (I) Localization of transgenically expressed Tmem81-3xFLAG-sfGFP and Izumo1-3xFLAG-sfGFP in live mature unactivated zebrafish sperm. A scheme of zebrafish sperm and the protein localization in sperm is shown on the left; the box indicates the zoomed view in the images. (J) KO strategy of mouse Tmem81 using two single-guide RNAs (sgRNAs) flanking its coding exon. A 849-bp deletion was introduced at the locus of Tmem81. Forward (Fw) and reverse (Rv) primers targeting the 5′ and 3′ untranslated regions (UTRs), respectively, were used for genomic PCR to detect the Tmem81 KO allele. (K) Gross appearance of Tmem81+/− and Tmem81−/− testes and testis weights in wild-type and Tmem81−/− males. (L) Sperm morphology in Tmem81+/− and Tmem81−/− males. (M) Sperm motility, progressive motility, and swimming velocity in wild-type and Tmem81−/− males. (N) In vitro fertilization between sperm and cumulus-intact (left) or cumulus-free eggs (right). Bar graphs represent mean ± SD. p values calculated by Student's t test (K and M). Abbreviations are as follows: Ud Spg, undifferentiated spermatogonia; A1-A2 Sg, A1-A2 differentiating spermatogonia; A3-B Sg, A3-A4-In-B differentiating spermatogonia; PreLe Sc, preleptotene spermatocytes; Le/Zy Sc, leptotene/zygotene spermatocytes; Pa Sc, pachytene spermatocytes; Di/Se Sc, diplotene/secondary spermatocytes; Early St, early round spermatids; Mid St, mid round spermatids; Late St, late round spermatids; VAP, velocity average path; VSL, velocity straight line; VCL, velocity curvilinear. |
Reprinted from Cell, 187(25), Deneke, V.E., Blaha, A., Lu, Y., Suwita, J.P., Draper, J.M., Phan, C.S., Panser, K., Schleiffer, A., Jacob, L., Humer, T., Stejskal, K., Krssakova, G., Roitinger, E., Handler, D., Kamoshita, M., Vance, T.D.R., Wang, X., Surm, J.M., Moran, Y., Lee, J.E., Ikawa, M., Pauli, A., A conserved fertilization complex bridges sperm and egg in vertebrates, 7066-7078.e22, Copyright (2024) with permission from Elsevier. Full text @ Cell