Fig 4
- ID
- ZDB-FIG-230407-24
- Publication
- Nelson et al., 2023 - Integration of cooperative and opposing molecular programs drives learning-associated behavioral plasticity
- Other Figures
- All Figure Page
- Back to All Figure Page
|
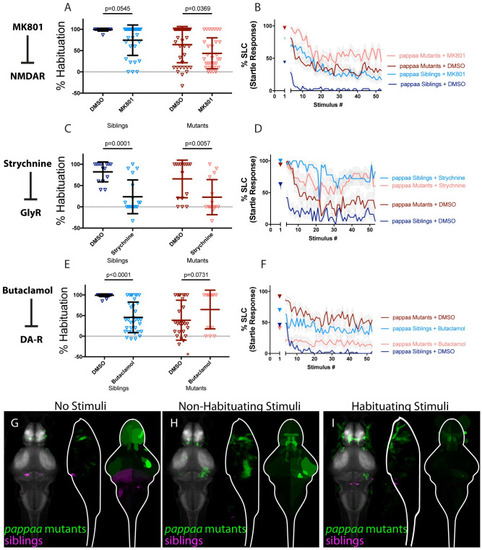
(A-B) MK-801 significantly enhances habituation learning deficits observed in pappaa mutant larvae, indicating that NMDA signaling and pappaa may act within parallel molecular or circuit pathways to regulate habituation learning (p = 0.0369, n = 39 DMSO-mutants, n = 32 MK-801 mutants; p = 0.0545, n = 16 DMSO-siblings, n = 31 MK-801 siblings; p = 0.7637 indicates non-significant interaction between drug treatment and genotype). (C-D) Strychnine significantly enhances habituation learning deficits observed in pappaa mutant larvae, indicating that glycine signaling and pappaa may act within parallel molecular or circuit pathways to regulate habituation learning (p = 0.0057, n = 16 DMSO-mutants, n = 15 Strychnine-mutants; p = 0.0001, n = 14 DMSO-Siblings, n = 18 Strychnine-siblings; p = 0.4286 indicates non-significant interaction between drug treatment and genotype). (E-F) Butaclamol does not significantly enhance habituation learning deficits observed in pappaa mutant larvae, but rather trends toward significantly restoring habituation learning (p<0.0001, n = 31 DMSO-siblings, n = 35 Butaclamol-siblings; p = 0.0731, n = 28 DMSO mutants, n = 13 Butaclamol mutants; p<0.0001 indicates significant interaction between drug treatment and genotype). *indicates a mutant+DMSO individual with % hab value below y-axis limit at -100% (G-I) Regions upregulated in pappaa mutants are indicated in green; regions downregulated in pappaa mutants are indicated in magenta. In all images, the left panel is a summed z-projection of the whole-brain activity changes. The middle panel is a summed x-projection of the whole brain activity changes. The right panel is a z-projection of the analyzed MAP-map. Patterns of neuronal activity are similar between “no stimuli” vs “non-habituating stimuli” vs. “habituating stimuli” (increased activity within the telencephalon and hypothalamus; decreased activity within multiple rhombencephalic loci). This pattern is somewhat inverted relative to that observed in Butaclamol-treated animals, consistent with a role for pappaa in regulating dopamine signaling. See also S1–S3 Tables for ROIs up- and down-regulated in each condition, as well as in independent replicates. |