Figure 4.
- ID
- ZDB-FIG-200523-11
- Publication
- Hailstone et al., 2020 - CytoCensus, mapping cell identity and division in tissues and organs using machine learning
- Other Figures
- All Figure Page
- Back to All Figure Page
|
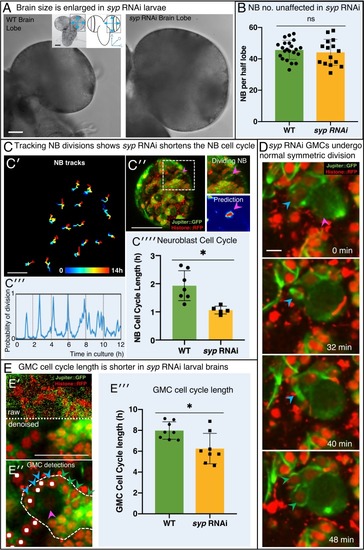
(A) Brightfield images of freshly isolated brains from third instar WT (OregonR) and syp RNAi larvae, respectively. Inserts in (A) show the region of the brain imaged and the measurements taken to compare brain size. (B) Chart comparing NB numbers showing that syp RNAi knockdown does not have a significant effect on NB number/brain (ns, p=0.77, t-test, WT n = 22; RNAi n = 15). NB were identified by Dpn labelling and the average count for a comparable volume of a single optic lobe CB region is shown. (C) Automated identification of NB division using CytoCensus: (C′) Tracking of NB centres, based on CytoCensus detections, over 14 h; (C′′) raw image showing single timepoint from live, 3D time-lapse, confocal imaging (insert = single dividing NB, showing CytoCensus prediction of a dividing NB); (C′′′) graph of division of a single tracked NB over 14 h; (C′′′′) average NB (6–9 NB/brain) cell cycle length is reduced in syp RNAi knockdown brains (p=0.004, Welch’s t-test, WT n = 7, syp RNAi n = 5 brains); (D) Sequence of confocal images from a typical 3D time-lapse movie showing that in syp RNAi brains, GMCs divide normally to produce two equal sized progeny that do not divide further. (E) Semi-automated analysis of GMC division by CytoCensus shows that GMC cell cycle length is reduced in syp RNAi brains. (E′) Single image plane taken from a 3D time-lapse, confocal image data set (imaged at one Z-stack/2 min). showing raw image data (top) and denoised (bottom). (E′′) CytoCensus GMC detections (cyan) with a single NB (magenta), and NB niche (dotted white line), shows GMCs are detected but neurons (green) are not. (E′′′) Plot of GMC cell cycle length, which is decreased in syp RNAi brains compared to WT (p=0.01, Welch’s t-test, n = 8 GMCs from three brains). Scale bars in (A) 50 µm; (C′) 20 µm; (C′′) 50 µm; (D) 5 µm; (E) 25 µm |