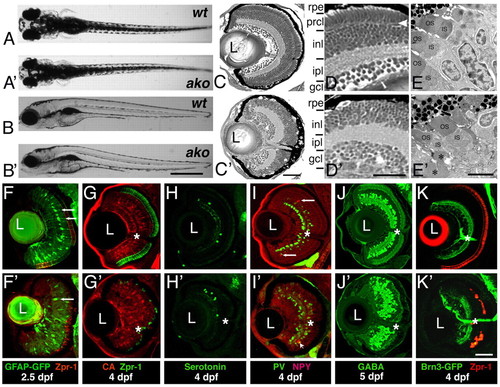
ale oko phenotype in the zebrafish retina. (A-B′) External phenotypes of akojj50 mutants (A′,B′), compared with their wild-type siblings (A,B). Dorsal (A,A′) and lateral (B,B′) views are shown at 5 dpf. (C-D′) Transverse sections of the ako (C′,D′) and wild-type (C,D) retinae at 4 dpf. (E,E′) Electron micrographs of wild-type (E) and mutant (E′) retinae at 3 dpf. OS, outer segment; IS, inner segment. Asterisks indicate abnormal mutant outer segments. (F-K′) Differentiation of retinal cells classes in ako mutants. Transverse cryosections through retinae of wild type (top) and akojj50 mutants (bottom). (F-G) Müller glia are visualized via the expression of a GFP transgene (F,F′, green, arrows) or by antibody staining against carbonic anhydrase (G,G′, red). Photoreceptors are stained with the Zpr-1 antibody (red in F,F′, green in G,G′). (H-J′) Transverse sections stained with antibodies to subpopulations of amacrine cells: anti-serotonin (H,H′, green), anti-neuropeptide Y (I,I′, red, arrows), anti-parvalbumin (I,I′, green) and anti-GABA (J,J′). (K,K) Sections through retinae of Tg(brn3c:mGFP) transgenic animals, which express GFP in ganglion cells (green). Photoreceptors are visualized with the Zpr-1 antibody (red). In G-K′, asterisks indicate the optic nerve. L, lens; rpe, retinal pigment epithelium; prcl, photoreceptor cell layer; inl, inner nuclear layer; ipl, inner plexiform layer; gcl, ganglion cell layer. Scale bars: 1 mm in A-B′; 40 μm in C,C′,F-K′; 30 μm in D,D′; 5 μm in E,E′.
|

