- Title
-
The zebrafish diwanka gene controls an early step of motor growth cone migration
- Authors
- Zeller, J. and Granato, M.
- Source
- Full text @ Development
|
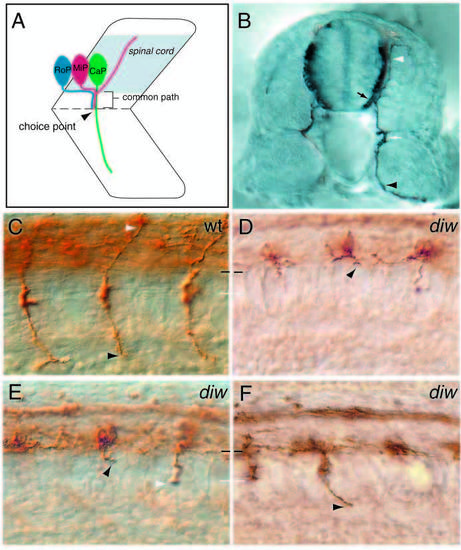
diwanka mutant embryos display severe motor axon defects. (A) Schematic drawing of a spinal hemisegment showing CaP, MiP and RoP neurons and their axonal projection (lateral view). (B) Cross-section through a 28 hpf embryo stained with the znp-1 antibody. Motor growth cones exit the spinal cord (black arrow) and migrate on the medial surface of the somites to the ventrally located choice point (white arrow) at the horizontal myoseptum. CaP (black arrowhead) axons continue further ventrally (white arrowhead), whereas MiP neurons form a branch to the dorsal somite (white arrowhead). Wild-type (C) and diwanka mutant (D-F) embryos at 23 hpf stained with znp-1 antibody. The common path extends between the lower end of the spinal cord (black line) and the horizontal myoseptum (white line). (C) Wild-type CaP (black arrowhead) and MiP (white arrowhead) axons have completed the common path and extended on their cell-type-specific paths. (D-F) Arrowheads points to diwanka mutant motor axons which failed to exit the spinal cord (D), stalled along the common path (black arrowhead in E), stalled at the distal end of the common path (white arrowhead), or have extended on the cell-type-specific path (F). EXPRESSION / LABELING:
PHENOTYPE:
|
|
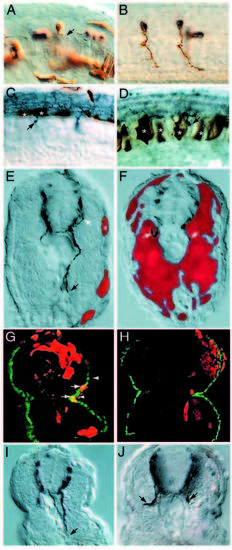
Motoneuron specification, notochord and floorplate development and spinal axonal projections are not affected in diwanka mutant embryos. (A,B) islet-1-positive cells in the ventral spinal cord consistent with the position of RoP neurons (black arrowhead) and MiP neurons (white arrowhead) are present in wild-type and mutant embryos. CaP, MiP and RoP motoneurons were identified by their dorsoventral and rostrocaudal position within the spinal cord and their soma size (see Table 1 for details). (C,D) type II collagen expression in the notochord (delineated by white arrowheads), in the floorplate (between black arrowhead and upper white arrowhead) and hypochord (between arrow and lower white arrowhead) is indistinguishable between wildtype and mutant embryos, suggesting that these cell types are not affected by mutations in the diwanka gene. (E,F) In wild-type and mutant embryos 3A10-positive commissural spinal interneurons (white arrowhead) extend their axons ventrally to the midline (black arrowhead), cross the midline and ascend on the contralateral side (arrow). Multiple focal planes were compiled to follow the entire axonal projections. EXPRESSION / LABELING:
|
|
diwanka is required for motor axon migration from the spinal cord to the somites. Stereotypic axonal projections of CaP (A), MiP (B) and RoP (C) neurons in wild-type embryos. White arrows point to the lower end of the spinal cord (out of focal plane) and black arrows point to the choice point. In diwanka mutants, all CaP axons (E) and MiP axons (F) extend normally within the spinal cord, but are severely affected as they migrate on the common path (black arrow). (G) All RoP axons in diwanka mutants complete the longitudinal path within the spinal cord, but most fail to exit the spinal cord and instead project further caudally within the spinal cord (arrowhead points to the growth cone). Note that the longitudinal projection of the mutant RoP axon is about twice the length of the corresponding projection of wild-type RoP in Fig. 4C. PHENOTYPE:
|
|
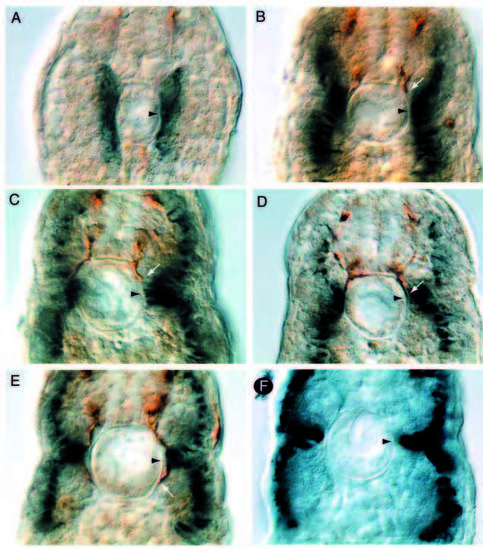
Analysis of chimeric mosaic embryos reveals that diwanka activity is required in adaxial cells. (A) A genotypically wild-type motoneuron (brown) which developed in diwanka mutant embryos and failed to exit the spinal cord (arrow). (B) Two genotypically mutant CaP motoneurons (brown) which developed in wild-type embryos and displayed normal axonal trajectories. (C,D) Wild-typederived floorplate (asterisks in C) or notochord cells (asterisks in D) failed to restore motor axonal trajectories. Arrows point to znp-1- positive motor growth cones, which failed to complete the common path. (E,F) Cross-section of mosaic diwanka embryos showing wildtype- derived cells (in red) and znp-1-positive motor axons in dark blue. (E) Small clones of genotypically wild-type cells in the lateral portion of the somite (asterisk) restored the motor axon projections of CaP (black arrow) and MiP (white arrow, only partially in focal plane). (F) In contrast, large clones of genotypically wild-type cells in the medial part of the somites failed to restore motor axon projections. (G) Cross-section showing colocalization between wildtype- derived cells (red) able to rescue the axonal phenotype in the right hemisegment and F59-positive adaxial cells (green). Arrows point to double-labeled cells (yellow), located between the lower end of the spinal cord (upper arrowhead) and the horizontal myoseptum (lower arrowhead). The rescued motor axon is shown in Fig. 4I. (H) Cross-section through a segment in which wild-type-derived cells (red) occupied most of the segment, but F59-positive adaxial cells (green) are of diwanka mutant origin. There is no colocalization between wild-type-derived cells (red) and F59-positive adaxial cells (green) and the motor trajectories were not rescued (see Fig. 4J). (I) DIC image of the cross-section shown in G. The arrow points to the rescued, znp-1-positive motor axon. (J) DIC image of the crosssection shown in H. Arrows point to mutant, znp-1-positive motor axons. |
|
Motor growth cone and adaxial cell migration coincide. (A-E) Cross-section of somitic segments (18-12) of wild-type embryos at 20 hpf stained with znp-1 antibody (brown) and F59 antibody (blue). White arrows point to the progressing growth cone and arrowheads point to distal end of the common path in the region of the future horizontal myoseptum. (F) Cross-section of somitic segment 14 of a diwanka mutant embryo at 24 hpf stained with F59 (blue), demonstrating that, in diwanka mutant embryos, the migrated adaxial cells are indistinguishable from those in wild-type siblings. |