- Title
-
Lactobacillus rhamnosus GG triggers intestinal epithelium injury in zebrafish revealing host dependent beneficial effects
- Authors
- Zhang, Z., Zhang, H.L., Yang, D.H., Hao, Q., Yang, H.W., Meng, D.L., Meindert de Vos, W., Guan, L.L., Liu, S.B., Teame, T., Gao, C.C., Ran, C., Yang, Y.L., Yao, Y.Y., Ding, Q.W., Zhou, Z.G.
- Source
- Full text @ Imeta
|
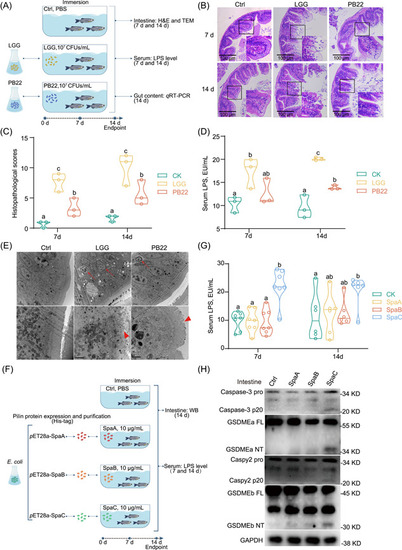
SpaC pilin subunit is responsible for zebrafish intestinal mucosa damage. (A) One‐month old zebrafish were immersed with either LGG or PB22 at 107 CFUs/mL for 7 or 14 days. (B) Representative intestinal histology images by H&E staining. Scale bar 100 μm. (C) Total histological score measuring the severity of the intestinal injury at 7 and 14 days (Day 7: |
|
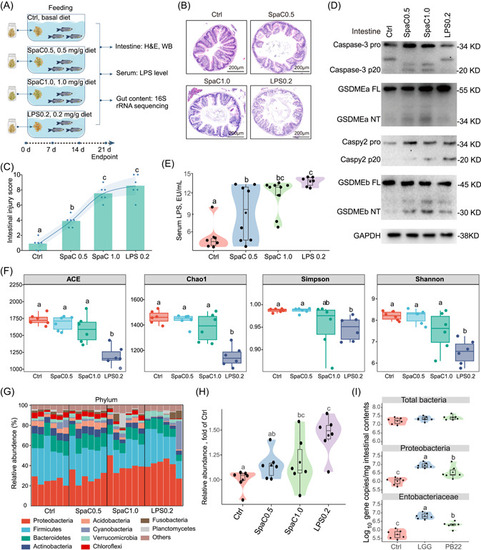
Dietary SpaC and LPS induce intestinal pyroptosis and gut microbial dysbiosis in zebrafish. (A) One‐month old zebrafish were fed with diets supplemented with 0.5 and 1.0 mg/g SpaC or diet supplemented with 0.2 mg/g LPS for three weeks. (B) Representative intestinal histology images by H&E staining. (C) Total histological score measuring the severity of the intestinal injury (Values are means. |
|
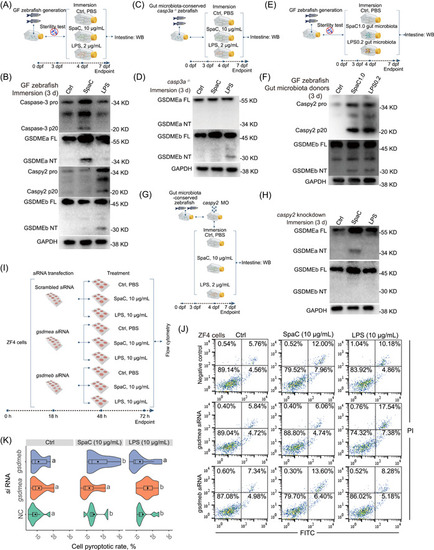
SpaC and LPS induce pyroptosis in gut microbiota‐independent and ‐dependent manners. (A) GF zebrafish (4‐dpf) were immersed with 10 μg/mL SpaC or LPS for 3 d. (B) A representative western blot analysis showing Caspase‐3 activation, GSDMEa cleavage, Caspy2 activation and GSDMEb cleavage in GF zebrafish. (C) The |
|
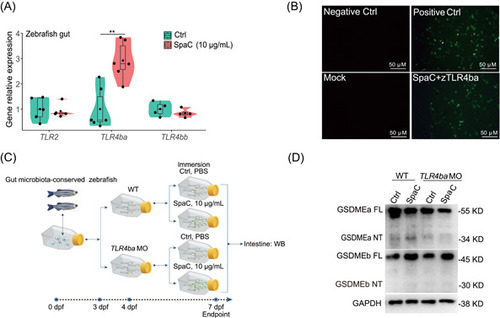
LGG SpaC pilin triggers intestinal pyroptosis by interacting with zebrafish TLR4ba. (A) One‐month old zebrafish were immersed with recombinant SpaC at concentrations of 10 μg/mL for Day 7. The relative mRNA expression of genes encoding |
|
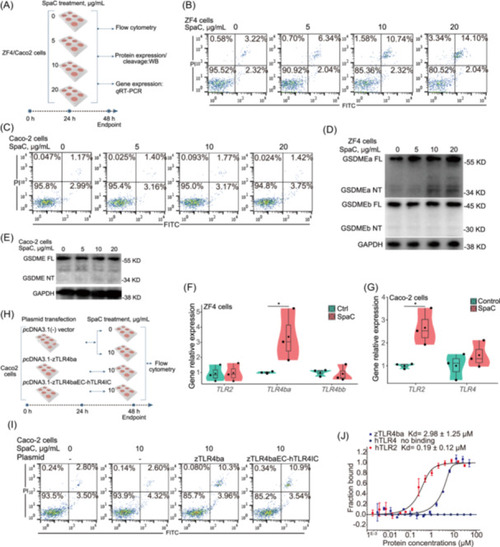
SpaC induces pyroptosis in zebrafish ZF4 cells rather than in human Caco‐2 cells. (A) Cells were treated with increasing concentrations of SpaC (0, 5, 10 and 20 μg/mL) for 24 h. (B) The flow cytometry results of ZF4 cells treated with recombinant SpaC protein in different dose for 24 h. (C) The flow cytometry results of Caco‐2 cells treated with recombinant SpaC protein in different dose for 24 h. (D) A representative western blot analysis showing GSDMEa and GSDMEb cleavage in ZF4 cells. (E) A representative western blot analysis showing GSDME cleavage in Caco‐2 cells. (F) The relative mRNA expression of genes encoding |
|
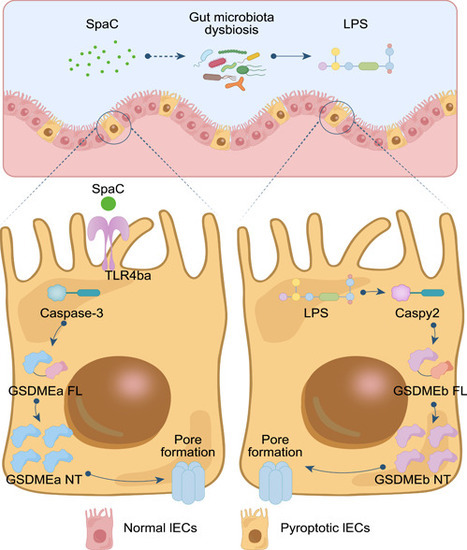
Illustration of the mechanisms underlying the activation of SpaC to intestinal pyroptosis. The SpaC pilin of LGG induces intestinal epithelium injury through a species‐specific activation of TLR4ba, which initiates Caspase‐3−GSDMEa pyroptosis and secondarily activates Gaspy2−GSDMEb pyroptosis via gut microbiota dysbiosis. |