- Title
-
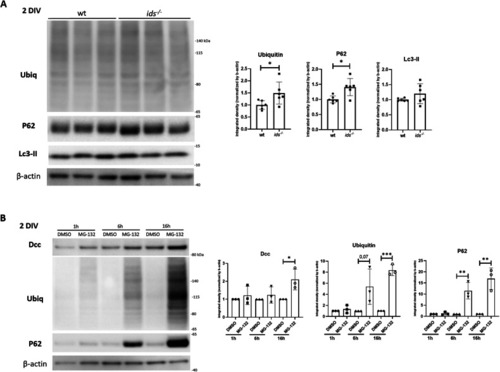
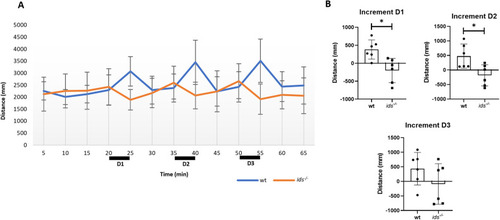
Mucopolysaccharidosis type II zebrafish model exhibits early impaired proteasomal-mediated degradation of the axon guidance receptor Dcc
- Authors
- Manzoli, R., Badenetti, L., Bruzzone, M., Macario, M.C., Rubin, M., Dal Maschio, M., Roveri, A., Moro, E.
- Source
- Full text @ Cell Death Dis.
|
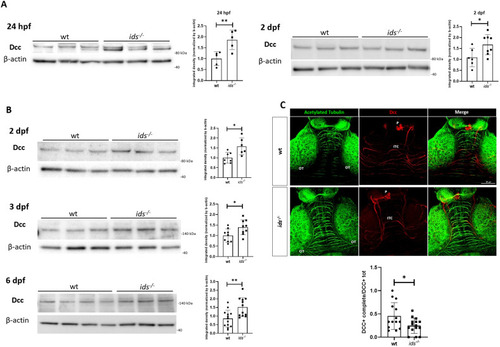
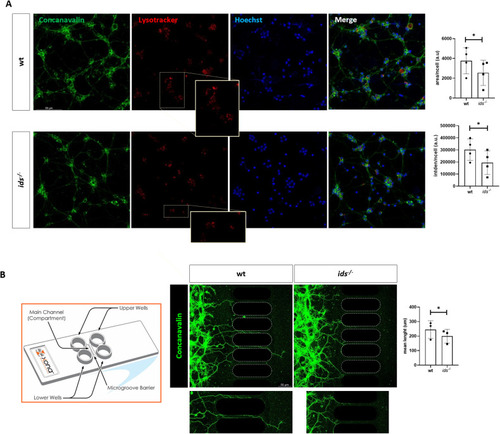
Dcc is significantly upregulated in |
|
|
|
|
|
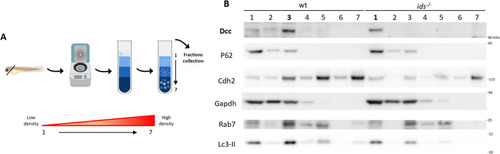
P62 protein levels are increased in Dcc-enriched fractions of |
|
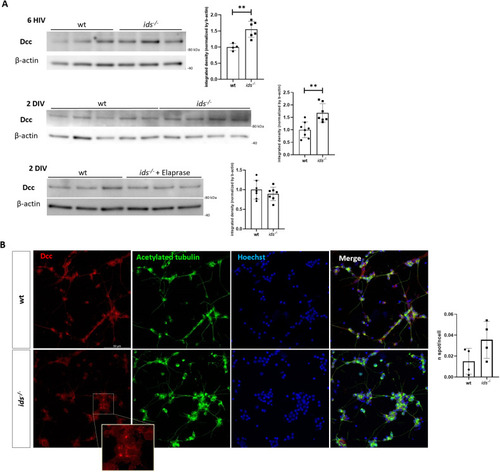
Proteasome inhibition in wild-type primary neurons results in Dcc upregulation. |
|
|