- Title
-
Isolation and proteomic study of fish liver lipid droplets
- Authors
- Sun, Y., Heng, J., Liu, F., Zhang, S., Liu, P.
- Source
- Full text @ Biophys Rep
|
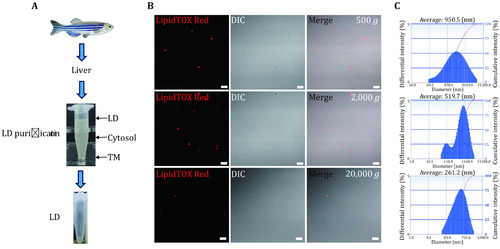
Isolation of LDs from zebrafish liver. |
|
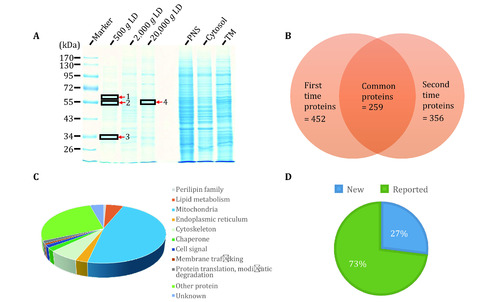
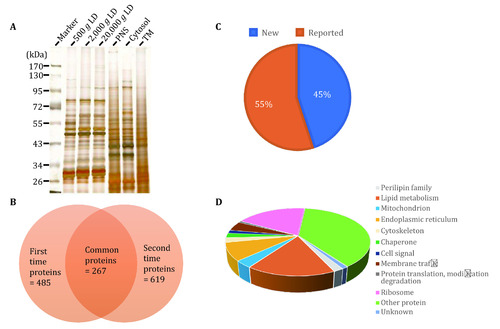
Proteomic analysis of isolated LDs from zebrafish liver. |
|
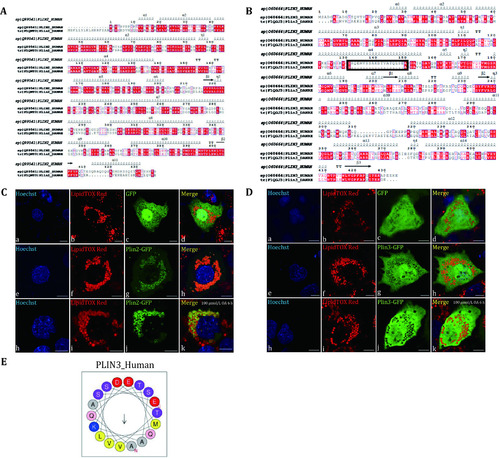
Localization of Plin2 and Plin3 of zebrafish in Huh7 cells. |
|
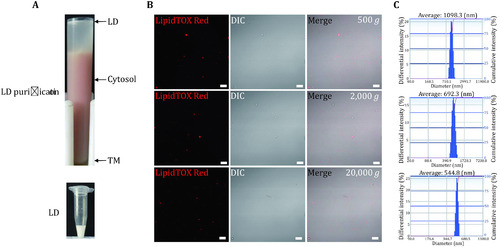
Isolation of LDs from |
|
Proteomic analyses of isolated LDs from |