- Title
-
Socially mediated shift in neural circuits activation regulated by synergistic neuromodulatory signaling
- Authors
- Clements, K.N., Ahn, S., Park, C., Heagy, F.K., Miller, T.H., Kassai, M., Issa, F.A.
- Source
- Full text @ eNeuro
|
|
|
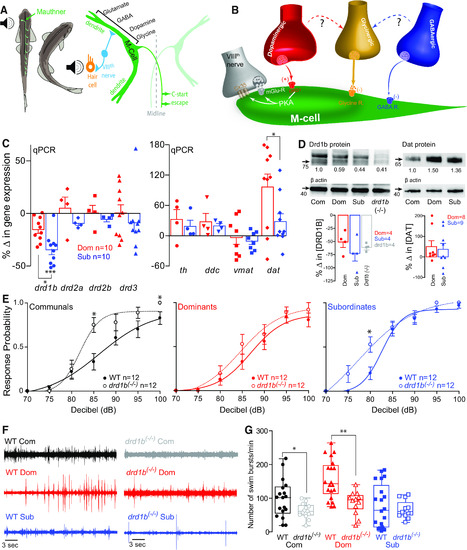
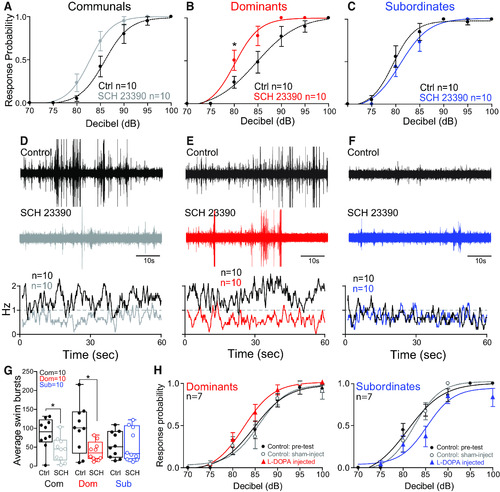
Dopaminergic modulation of the escape and swim circuits is socially regulated. |
|
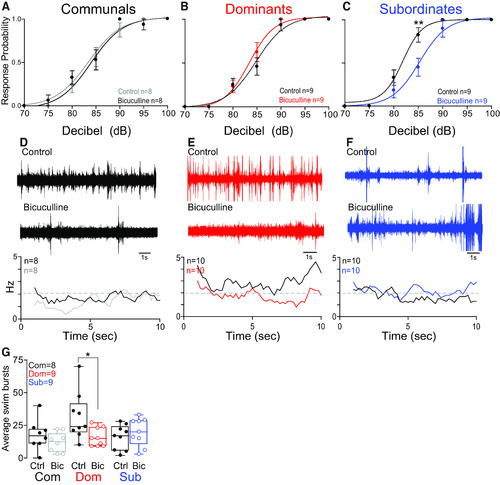
GABAergic modulation of the escape and swim circuits is socially regulated. |
|
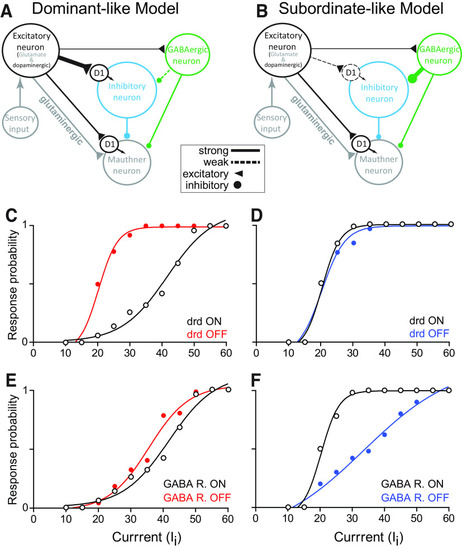
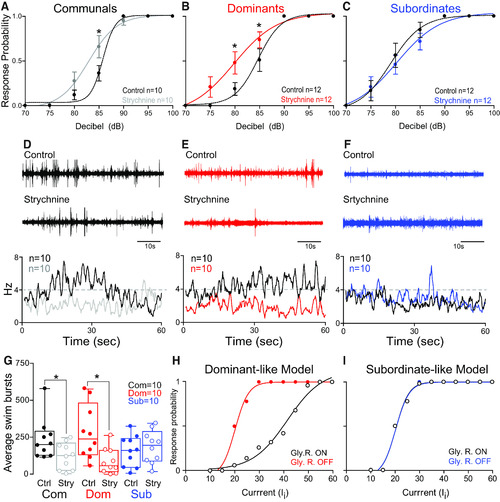
Neurocomputational model. Schematic of dominant-like ( |
|
Glycinergic modulation of the escape and swim circuits is socially regulated. |
|
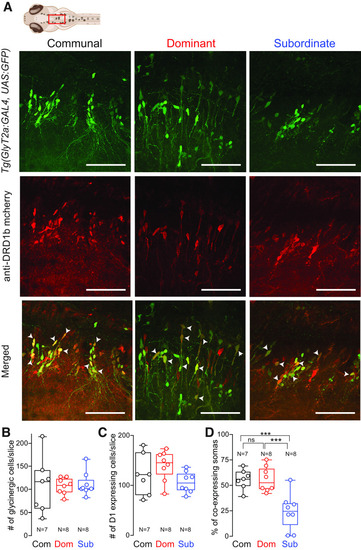
Status-dependent expression of Drd1b in hindbrain glycinergic neurons. |
|
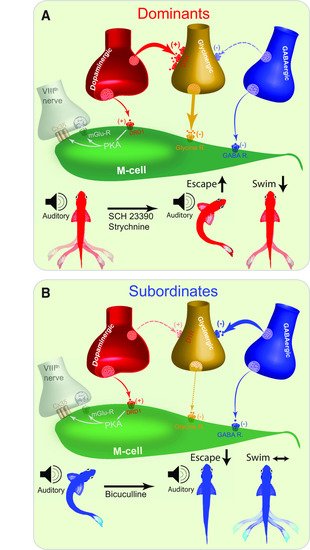
Proposed model of socially mediated shift in synaptic reconfiguration underlies synergistic modulation of the motor circuits. |