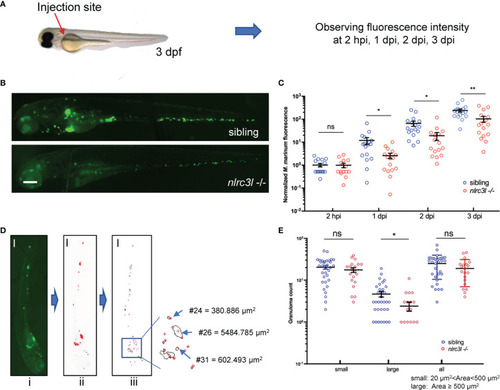
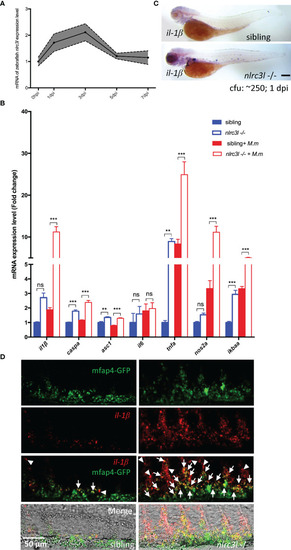
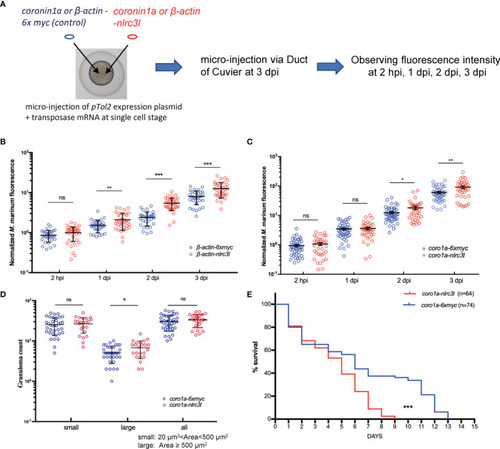
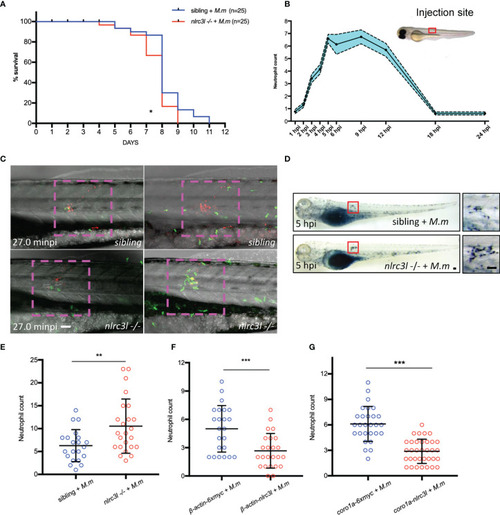
- Title
-
Negative Regulator Nlrc3-like Maintain the Balanced Innate Immune Response During Mycobacterial Infection in Zebrafish
- Authors
- Niu, L., Luo, G., Liang, R., Qiu, C., Yang, J., Xie, L., Zhang, K., Tian, Y., Wang, D., Song, S., Takiff, H.E., Wong, K.W., Fan, X., Gao, Q., Yan, B.
- Source
- Full text @ Front Immunol
|
|
|
|
|
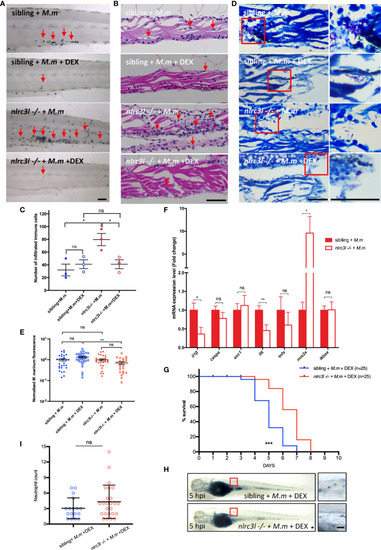
The impact of |
|
|
|
|
|
|
|
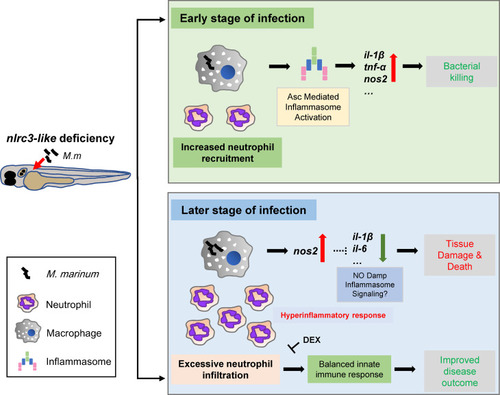
Model for the impact of negative regulator NLR |