- Title
-
Egr1 is necessary for forebrain dopaminergic signaling during social behavior
- Authors
- Tallafuss, A., Stednitz, S.J., Voeun, M., Levichev, A., Larsch, J., Eisen, J., Washbourne, P.
- Source
- Full text @ eNeuro
|
|
|
|
|
|
|
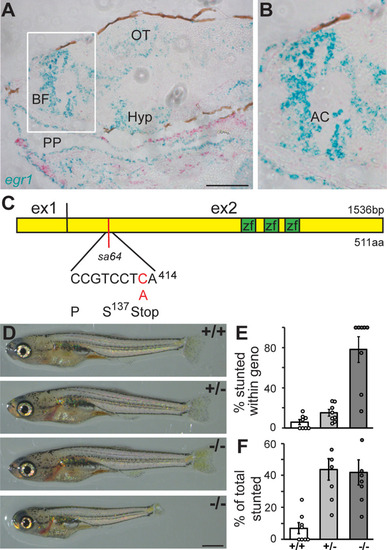
Egr1 is necessary for TH2 expression. |
|
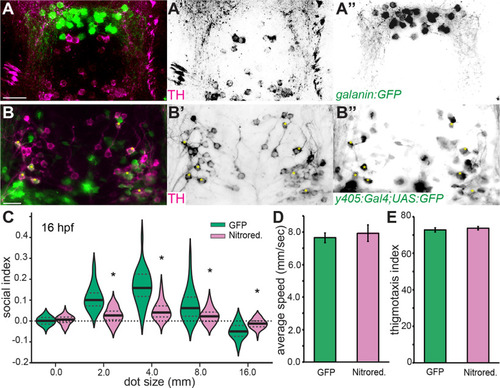
Egr1 is necessary for TH-positive neuron number in BF. |
|
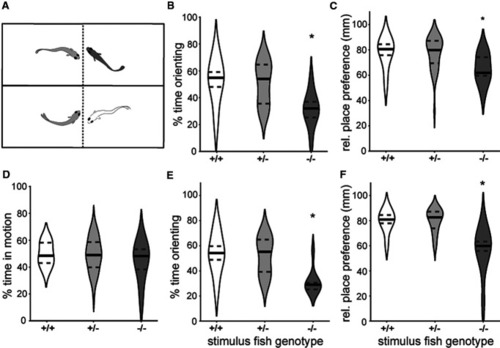
TH-positive neurons in PPa are necessary for social behavior. |
|
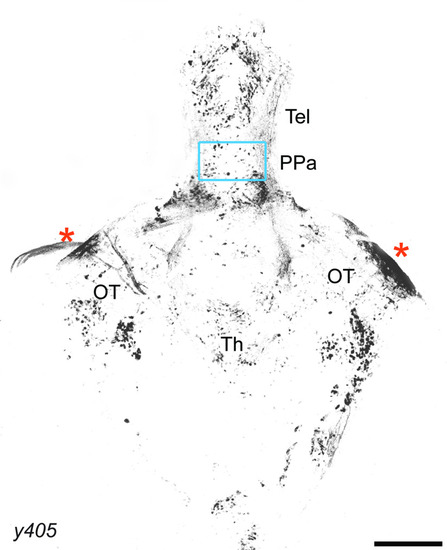
Whole-brain expression of |