- Title
-
Drivers of Sinoatrial Node Automaticity in Zebrafish: Comparison With Mechanisms of Mammalian Pacemaker Function
- Authors
- Stoyek, M.R., MacDonald, E.A., Mantifel, M., Baillie, J.S., Selig, B.M., Croll, R.P., Smith, F.M., Quinn, T.A.
- Source
- Full text @ Front. Physiol.
|
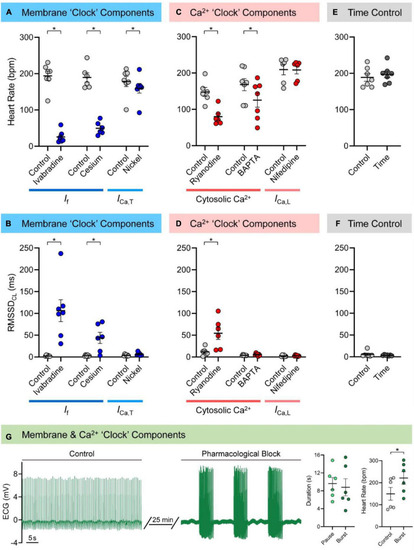
Mechanisms of zebrafish sinoatrial node (SAN) automaticity. Effects of pharmacological block of membrane (40 min of 3 μM ivabradine hydrochloride for intracellular block of “funny” current, |
|
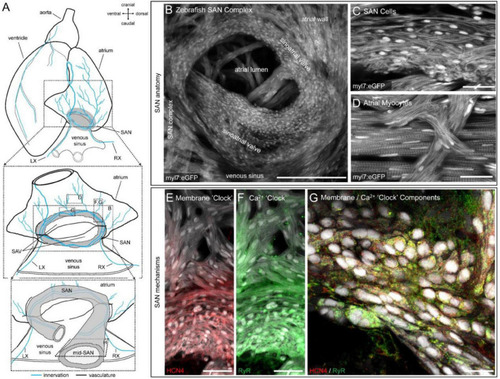
Structure and channel expression in the zebrafish sinoatrial node (SAN). Schematic of the zebrafish heart illustrating the location of the images in B-H |
|
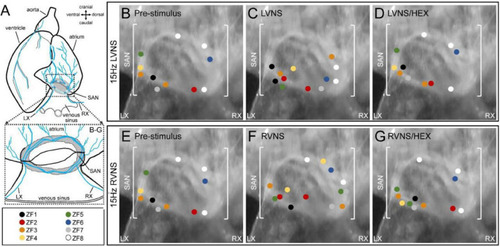
Effect of vagal nerve stimulation on leading pacemaker site of the zebrafish sinoatrial node (SAN). Schematic of the zebrafish isolated heart showing the location of the SAN and the intact left (LX) and right (RX) cardiac branches of the vagal nerve |
|
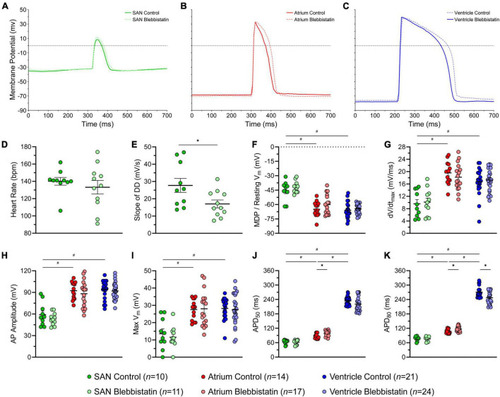
Effects of blebbistatin on the zebrafish sinoatrial node (SAN), atrial, and ventricular action potential (AP). Representative AP |
|
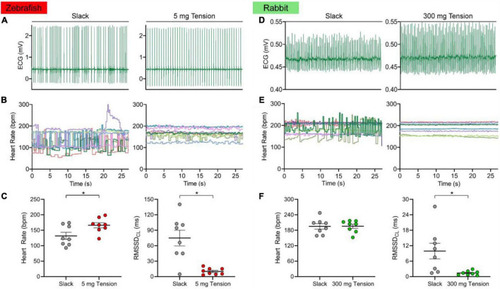
Effect of mechanical preload on zebrafish and rabbit sinoatrial node automaticity. Representative electrocardiogram (ECG) recording of zebrafish |
|
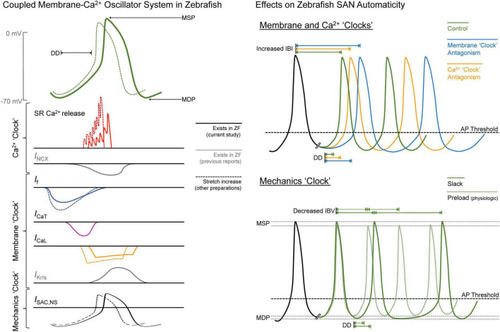
Figure 6. Summary of the drivers of zebrafish sinoatrial node (SAN) automaticity. AP, action potential; DD, diastolic depolarisation; ICa,L, L-type Ca2+ current; ICa,T, T-type Ca2+ current; IBI, inter-beat interval; IBV, inter-beat variation; If, “funny” current; IKr/s, rapid/slow delayed rectifier K+ current; INCX, Na+-Ca2+ exchanger current; ISAC,NS, cation non-selective stretch-activated channels; MDP, maximum diastolic potential; MSP, maximum systolic potential; RyR, ryanodine receptors; SR, sarcoplasmic reticulum; ZF, zebrafish. |