- Title
-
The perinuclear region concentrates disordered proteins with predicted phase separation distributed in a 3D network of cytoskeletal filaments and organelles
- Authors
- do Amaral, M.J., de Andrade Rosa, I., Andrade, S.A., Fang, X., Andrade, L.R., Costa, M.L., Mermelstein, C.
- Source
- Full text @ BBA Molecular Cell Research
|
An intricated network of cytoskeletal filaments and several organelles are found in the nuclear space of eukaryotic cells. (A and B) COS-7 cells were extracted and analyzed under transmission electron microscopy. Note the presence of a dense network (in A and B) of cytoskeletal filaments (Fi) linked to the nucleus (Nu). In the higher magnification (B) it is possible to see several protein aggregates (Ag) attached to the cytoskeletal network. Scale bars in A and B = 500 ηm. (C–G) 2-month-old mouse cardiac tissues were processed for transmission electron microscopy and images show mitochondria (Mt), Golgi apparatus (G), vesicles (Ve), myofibers (Mf), and cytoskeletal filaments (Fi) in proximity with the nucleus (Nu). Arrow in (C) shows cytoskeletal filaments in the perinuclear space, arrow in (D) shows mitochondria in close contact with the outer nuclear membrane, and arrow in (G) points to the Z-disk of a myofibril in the vicinity of the nucleus. Scale bars in C-G = 2 μm. N = 3 independent experiments. |
|
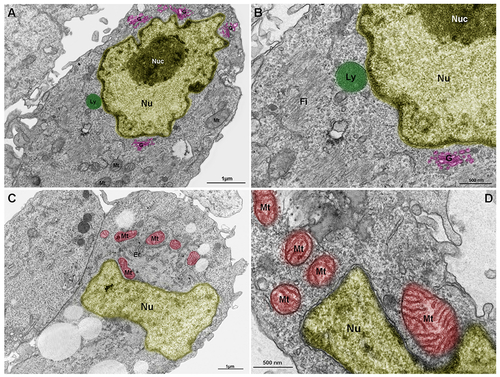
Lysosomes, mitochondria and Golgi are components of the nuclear cloud. Embryonic chick pectoral muscle was processed for transmission electron microscopy and organelles were digitally colored to facilitate the visualization. Images show lysosomes (Ly, in green in A and B), mitochondria (Mt, in red in C and D), endoplasmic reticulum (Er in C), and Golgi (G, in pink in A and B) in proximity with the outer nuclear membrane (Nu). The highly packed nuclear compartment nucleoli (Nuc, in dark yellow) are seen within the nucleus (light yellow) of muscle cells (in A and B). Some lysosomes (in A and B) and mitochondria (in C and D) seem to be adhered to the nuclear surface. Bars in A and C = 1 μm, and bars in B and D = 500 nm. N = 4 independent experiments. |
|
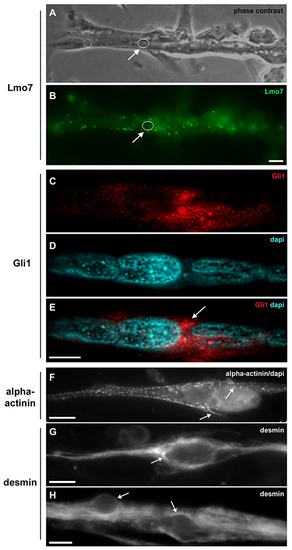
Lmo7, Gli1, alpha-actinin and desmin are concentrated in the nuclear cloud in skeletal muscle fibers. Primary cultures of chick myogenic cells were labeled with antibodies against Lmo7 (B), Gli1 (C–E), alpha-actinin (F) and desmin (G–H), and with the nuclear dye DAPI (D–F). A 72-h chick multinucleated myotube was visualized under phase contrast microscopy (A) and under fluorescence microscopy to show the localization of Lmo7 (green, in B). Arrows in A and B point to Lmo7 distribution near the nuclear surface of a myotube. White open circles (in A and B) mark the region of one nucleus surrounded by Lmo7-positive aggregates. Scale bar in A = 10 μm. Gli-1 (red, in C and E) localizes at the perinuclear region of a 72-h multinucleated myotube (arrow in E). A merged image (with Gli1 and DAPI) is shown in E. Scale bar in E = 5 μm. The intermediate filament desmin and the sarcomeric protein alpha-actinin accumulate at the perinuclear region of mononucleated myoblasts (F and G) and multinucleated myotubes (H). A merged image of sarcomeric alpha-actinin and DAPI is shown in (F). Note the punctate distribution of alpha-actinin (arrows in F) and the continuous distribution of desmin filaments (arrows in G and H) in the juxtanuclear region of cells. Scale bars in F and H = 10 μm, and in G = 5 μm. n = 4 independent experiments. |
|
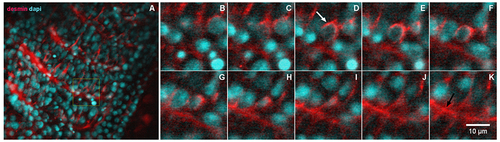
Desmin accumulates at the perinuclear region of early somites in zebrafish embryos. 24-h zebrafish embryos were labeled with an antibody against the muscle-specific intermediate filament protein desmin (red, in A–K) and with the nuclear dye DAPI (cyan, in A–K). Higher magnifications of the area marked in the inset in (A) are shown in images (B–K). Immunofluorescence confocal images of different focal planes (1 μm apart, in B–K) show the perinuclear localization of desmin (white arrow in D) in somite 28 at the most caudal region of a 24-h zebrafish embryo. Desmin is also found at the septa between adjacent muscle somites 28 and 29 in zebrafish embryos (black arrow in K). Scale bar in K = 10 μm. N = 4 independent experiments. |
|
Some proteins of the perinuclear region show the unusual chemical characteristics of IDPs. (A) The charge-hydropathy analysis of the human perinuclear proteins retrieved from the UniProt database (purple squares) and literature (green circles). Inset: globular folded proteins represented as blue circles and natively unfolded proteins as red dots. (B) Zoomed area marked by dashed line from “A”. (C) List of perinuclear proteins with the exquisite charge-hydropathy of IDPs, numbers refer to “B”. Plot described by [29] using 275 globular proteins (blue circles) and 91 IDPs (red circles) that enable differentiation (black line) by their charged and hydrophobic nature at pH 7.0 and obtained by PONDR (available at http://www.pondr.com/). |
|
Perinuclear IDR-containing proteins with phase separation ability. (A) Venn diagram (analyzed with BioVenn, available at http://www.biovenn.nl) showing the overlap between predictions by catGRANULE (score ≥ 0.5), PScore (score ≥ 4.0) and perinuclear proteins with peer-reviewed published evidence of phase behavior (in vitro and/or in vivo). (B) STRING network of functional and physical protein-protein interactions. Top: perinuclear LLPS-IDR-containing proteins with 43 members. Bottom: LLPS-IDR-containing proteins with 74 members. Proteins highlighted in cyan are potential hubs using this classification. (C) Combined STRING (only physical interaction shown) and UniProt keywords evaluation of NA-related and cytoskeleton-related processes. Top: LLPS-IDR-containing proteins. Bottom: non-LLPS-IDR-containing proteins. Evidence from literature is assigned with PMID. Legend is shown in the middle. |
|
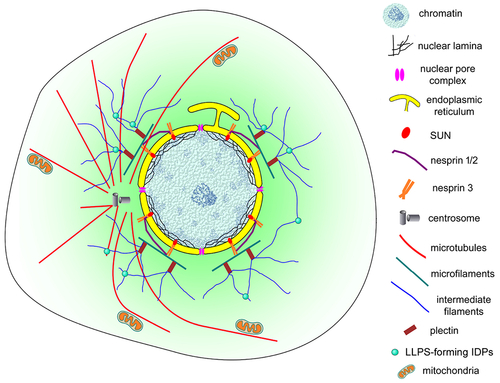
Schematic representation of the nuclear cloud of eukaryotic cells. This model illustrates the complex organization of the perinuclear space (shown in light green) of eukaryotic cells. An intricate 3D network of cytoskeletal filaments is found attached to the nuclear membrane (yellow). Several proteins participate in this organization: cytoskeletal (microfilaments in green, microtubules in red and intermediate filaments in blue) and cytoskeletal associated proteins (plectin, alpha-actinin, and others), nesprins, SUNs, among many others. Dynamic biomolecular condensates drove by intrinsically disordered proteins (IDPs), have a higher propensity to be assembled (small green spheres). Different organelles and cellular structures are also present in the nuclear could: mitochondria (orange), lysosomes, endoplasmic reticulum, Golgi, vesicles, centrosome (grey), and myofibers (in muscle cells). |
Reprinted from Biochimica et biophysica acta. Molecular cell research, 1869, do Amaral, M.J., de Andrade Rosa, I., Andrade, S.A., Fang, X., Andrade, L.R., Costa, M.L., Mermelstein, C., The perinuclear region concentrates disordered proteins with predicted phase separation distributed in a 3D network of cytoskeletal filaments and organelles, 119161, Copyright (2022) with permission from Elsevier. Full text @ BBA Molecular Cell Research