- Title
-
Spectral inference reveals principal cone-integration rules of the zebrafish inner retina
- Authors
- Bartel, P., Yoshimatsu, T., Janiak, F.K., Baden, T.
- Source
- Full text @ Curr. Biol.
|
Measuring high-spectral resolution tuning curves in zebrafish bipolar cells (A) Schematic of the larval zebrafish retina, with cone terminals in the outer retina and bipolar cell (BC) terminals in the inner retina highlighted. (B) Mean calcium responses of red-, green-, blue-, and UV-cone terminals to a series of 13 spectrally distinct widefield flashes of light as indicated (data from Yoshimatsu et al. (C–F) Illustration of recording strategy for BC terminals in the inner plexiform layer (IPL) and exemplary results. An optical triplane approach (C, top) was used to simultaneously record from three planes of larval zebrafish BC terminals expressing SyjGCaMP7b by way of two-photon imaging coupled with remote focusing ( Zebrafish larva schematic (A) by Lizzy Griffith. See also |
|
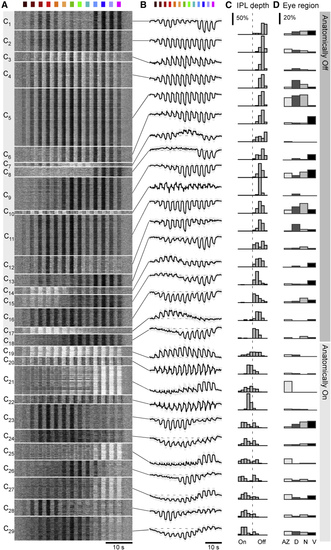
Clustering into 29 functional BC types Overview of the result from unsupervised clustering of all BC data recorded as shown in |
|
Reconstructing bipolar cell responses from cones (A–E) Summary of the reconstruction strategy for example cluster C22 (for details, see (F) As (A)–(E) but showing only the weights (top), cone totals (middle), and full reconstructions (bottom) for another four example clusters (from left: C1; C15; C14; and C25). Further detail on reconstructions is shown in |
|
A functional overview of cone bipolar cell mappings Overview of all BC-cluster means (A, gray traces; cf. |
|
Major trends in cone weights and spectral tunings (A and B) Histograms of all weights associated with inputs to each of the four cones across all clusters, independent of temporal-component types (A) and, correspondingly, histograms of all weights associated with temporal components, independent of cone type (B). Near-zero weights (abs(w) < 0.5) are graphically de-emphasized for clarity. All weights contributed equally to these histograms, independent of the size of their corresponding cluster. (C–E) Scatterplots of all clusters’ weights associated with each cone plotted against each other as indicated. Large symbols denote the mean weight associated with each cone and cluster across all four temporal components (i.e., one symbol per cluster), while small symbols denote each weight individually (i.e., four symbols per cluster, corresponding to Ltr, Lsus, Dtr, and Dsus). The remaining three possible cone correspondences (G:B, G:U, and B:U) are shown in (F–K) Peak-normalized “bulk” spectral tuning functions of all 29 clusters, grouped into six categories as indicated. The strength of each line indicates the numerical abundance of ROIs belonging to each cluster (darker shading = larger number of ROIs; exact number of ROIs contributing to each cluster are listed in (L) Cones’ spectral tuning functions, with approximate zero crossings (blue and green cones) and zero positions (red and UV cones) graphically indicated. (M and N) Histograms of zero crossings across all BC clusters, incorporating the abundance of ROIs belonging to each cluster. Shown are crossings of bulk spectral tunings functions (M; cf. F–H) and of spectral tuning functions that were computed for each temporal component individually, as indicated (see also |
|
Cone-weight distribution across the inner plexiform layer Two-dimensional histograms of weights (x axes) associated with each cone resolved by IPL position (y axes). Brighter colors denote increased abundance. For simplicity, the weights associated with the light (Ltr and Lsus) and dark components (Dtr and Dsus), are combined in (A) and (B), respectively. Moreover, near-zero weights are not shown (central white bar in all panels). The thick white dotted lines indicate approximate expected distribution of weights based on traditional “On-Off” lamination of the inner retina. By each panel’s side, instances where this expectation is violated are highlighted as “polarity violation.” |
|
Possible links across vertebrate retinal color circuits Conceptual summary schematics of retinal circuits for color vision in zebrafish (A); dichromatic mammals, such as many rodents (B); and some trichromatic old-world monkeys, such as humans (C). The colored “graphs” indicate approximate spectral tuning functions of retinal neurons in a given layer, as indicated. |