- Title
-
Phorbol 12-Myristate 13-Acetate Induced Toxicity Study and the Role of Tangeretin in Abrogating HIF-1α-NF-κB Crosstalk In Vitro and In Vivo
- Authors
- Chang, S.N., Dey, D.K., Oh, S.T., Kong, W.H., Cho, K.H., Al-Olayan, E.M., Hwang, B.S., Kang, S.C., Park, J.G.
- Source
- Full text @ Int. J. Mol. Sci.
|
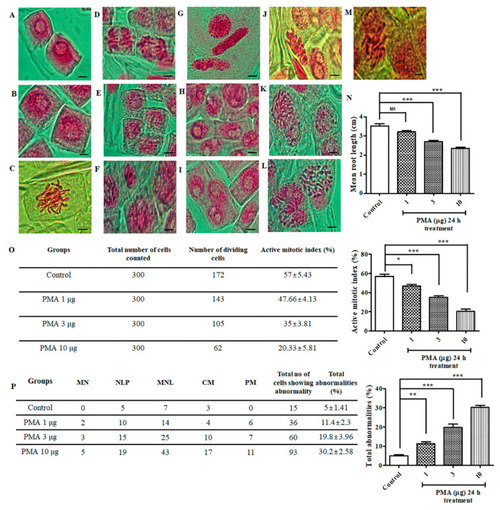
Chromosomal aberrations induced by phorbol 12-myristate 13-acetate (PMA) in the root tip meristem of |
|
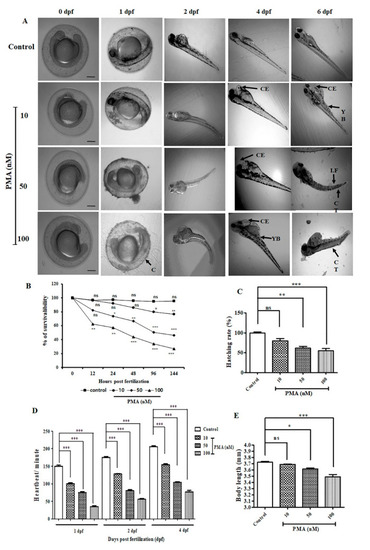
PMA exerted various deformities in zebrafish embryo and larvae ( |
|
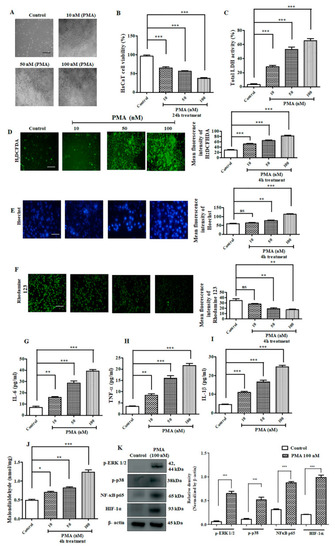
Effect of PMA on immortalized human keratinocyte (HACaT) cells. ( |
|
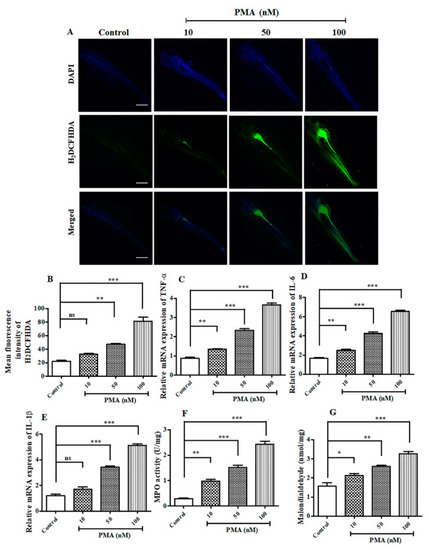
PMA induced elevated reactive oxygen species (ROS) and inflammatory response in Zebrafish larvae. ( |
|
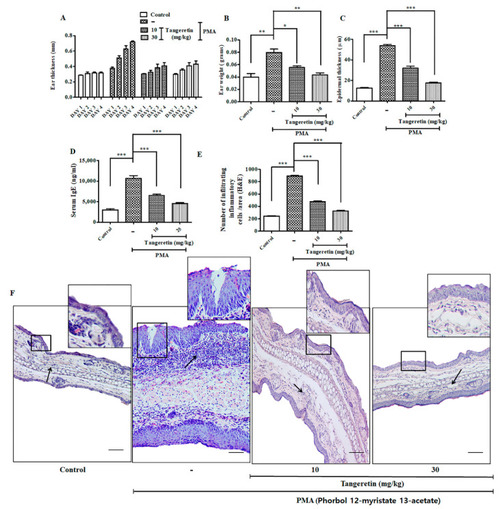
Tangeretin (TAN) remarkably ameliorated PMA induced epidermal hyperplasia intra-epidermal neutrophilic abscesses (mice, |
|
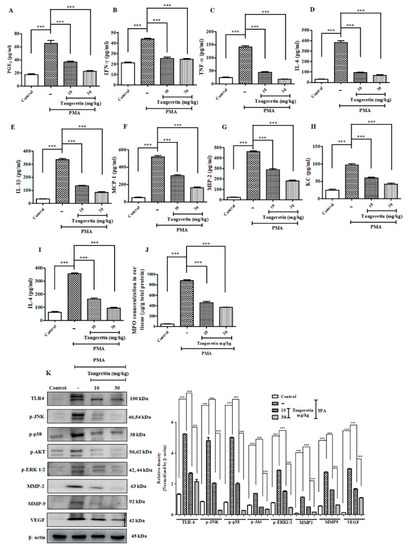
TAN exhibited potent anti-inflammatory response and blockaded cell proliferation pathway. Measurement of different inflammatory markers on mice after PMA treatment estimated by ELISA analysis. ( |
|
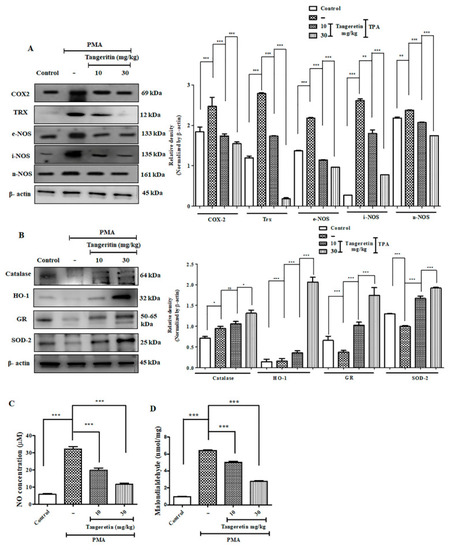
TAN neutralized PMA induced elevated ROS production by promoting antioxidant response. ( |
|
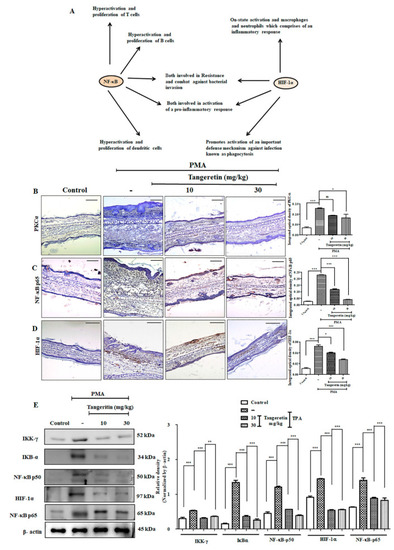
TAN blocks PMA induced hypoxia-inducible factor 1-alpha (HIF-1α) and nuclear factor kappa-light-chain-enhancer of activated b cells (NF-κB) inflammatory crosstalk. ( |
|
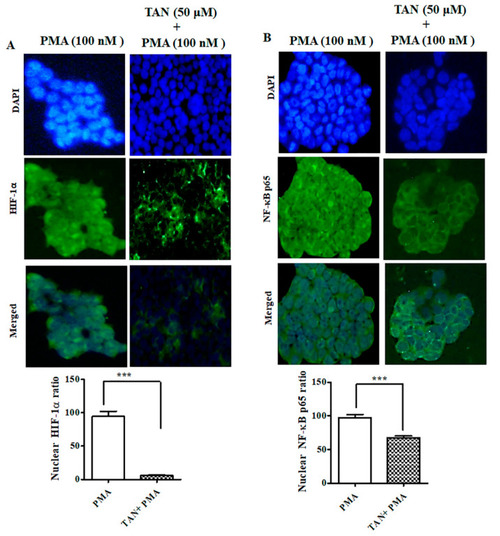
TAN inhibited PMA induced NF-κB-p65 and HIF-1α nuclear translocation on HaCaT cells. ( |
|
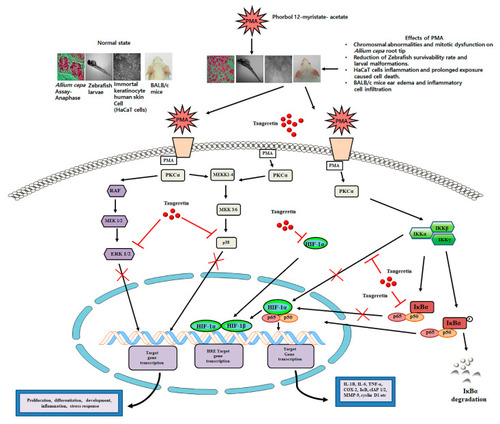
A schematic illustration of the role of tangeretin in counteracting PMA-induced inflammatory response on in vitro and in vivo model systems. |