- Title
-
Light-triggered switching of liposome surface charge directs delivery of membrane impermeable payloads in vivo
- Authors
- Arias-Alpizar, G., Kong, L., Vlieg, R.C., Rabe, A., Papadopoulou, P., Meijer, M.S., Bonnet, S., Vogel, S., van Noort, J., Kros, A., Campbell, F.
- Source
- Full text @ Nat. Commun.
|
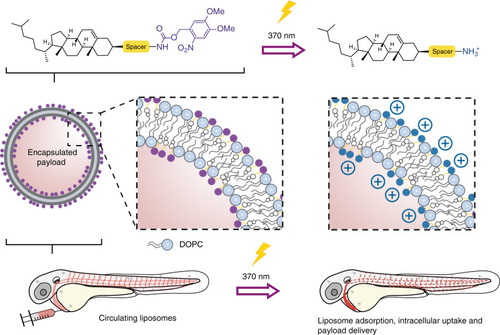
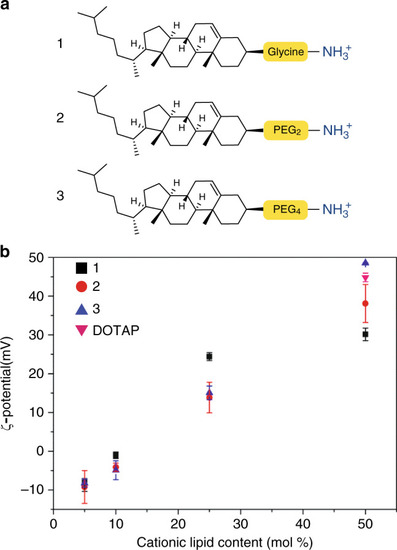
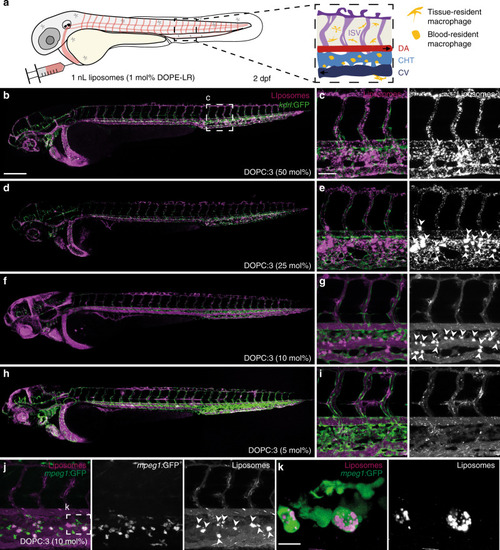
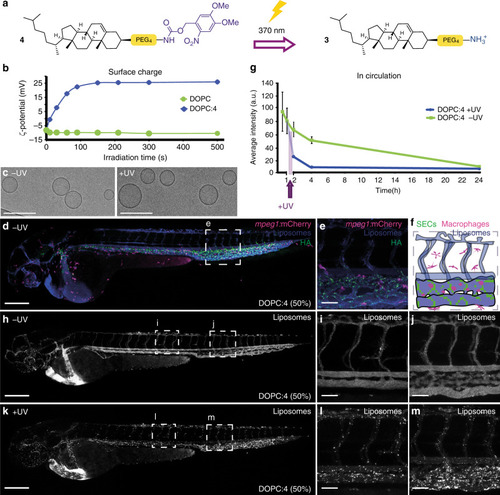
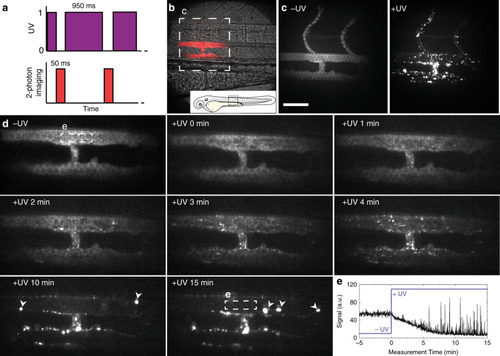
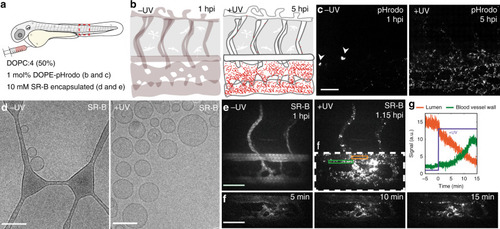
Prior to light activation, charge neutral, photoactive liposomes freely circulate throughout the vasculature of a zebrafish embryo and do not interact with RES cell types, or any other cell type, of the embryonic fish. Upon light irradiation and photolysis of photocaged, cholesterylamine lipids, rapid surface charge switching, from neutral to cationic, leads to non-specific adsorption of liposomes across the endothelium of the embryo, liposome uptake and intracellular delivery of liposome-encapsulated, membrane impermeable payloads. DOPC 1,2-dioleoyl- |
|
|
|
|
|
|
|
|
|
|
|
|