- Title
-
Disruption of the kringle 1 domain of prothrombin leads to late onset mortality in zebrafish
- Authors
- Grzegorski, S.J., Hu, Z., Liu, Y., Yu, X., Ferguson, A.C., Madarati, H., Friedmann, A.P., Reyon, D., Kim, P.Y., Kretz, C.A., Joung, J.K., Shavit, J.A.
- Source
- Full text @ Sci. Rep.
|
Peptide sequence alignment shows strong conservation of prothrombin across a broad range of species. Sequences shown include the conserved domains. Numbering begins at the first residue after the propetide according to the prothrombin numbering scheme. Sequences are shaded to indicate degree of conservation. Green colored residues indicate conserved cysteines. Red colored residues represent amino acids altered by mutagenesis (Δ15; C138A) with the affected paired cysteines highlighted in yellow. Numbers above alignment indicate paired cysteines in human prothrombin. |
|
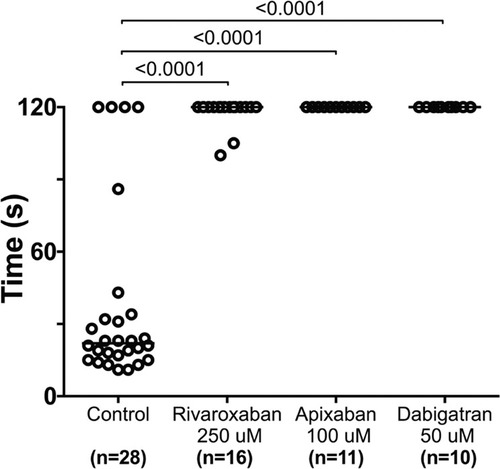
Direct oral anticoagulants prevent occlusive thrombus formation in zebrafish. 5 dpf larvae were exposed to chemical inhibitors of thrombin (dabigatran) and FXa (apixaban, rivaroxaban) for 24 hours. This resulted in the inability to form occlusive venous thrombi at 6 dpf in wild-type larvae following laser-mediated endothelial injury. PHENOTYPE:
|
|
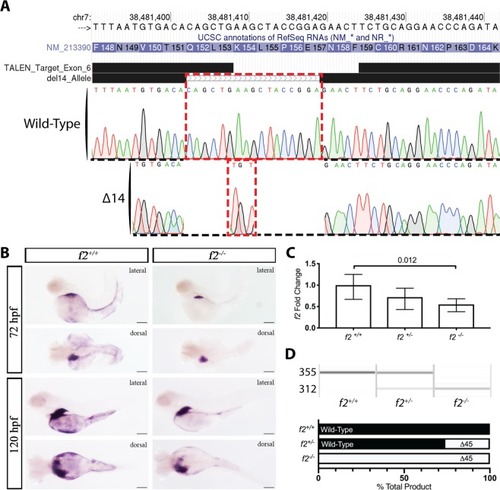
Genome editing creates a 14 bp genomic deletion with a resulting decrease in mRNA expression. ( EXPRESSION / LABELING:
PHENOTYPE:
|
|
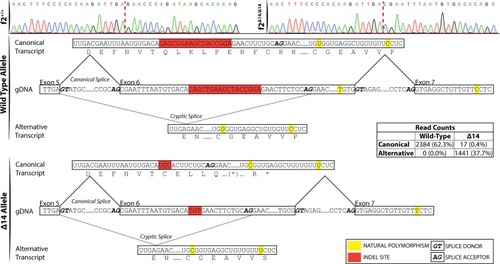
Single molecule real time sequencing of |
|
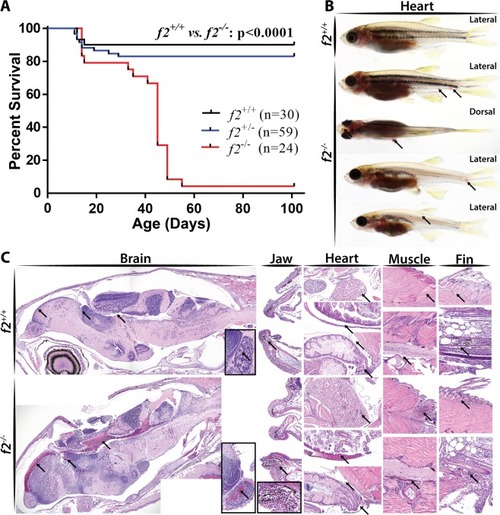
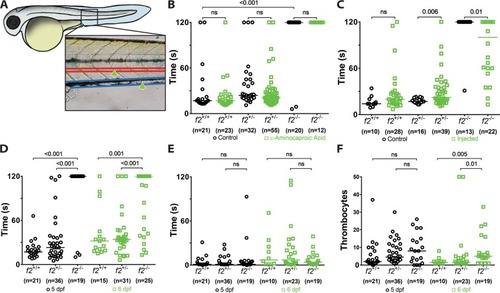
Loss of prothrombin results in early lethality due to hemorrhage. ( PHENOTYPE:
|
|
Loss of thrombin activity results in defects in secondary hemostasis. ( PHENOTYPE:
|
|
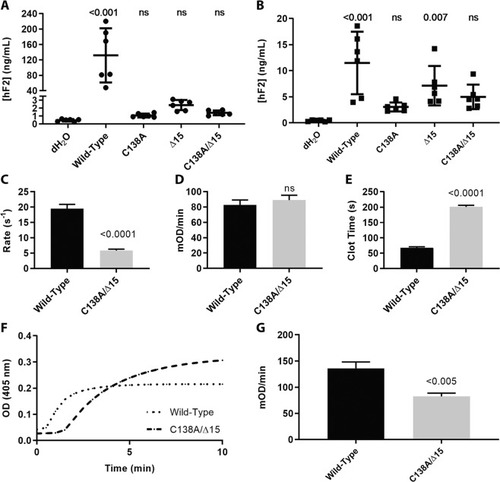
Expression and activation of prothrombin variants and resulting activity of thrombin upon activation. ( |