- Title
-
Sec14l3 potentiates VEGFR2 signaling to regulate zebrafish vasculogenesis
- Authors
- Gong, B., Li, Z., Xiao, W., Li, G., Ding, S., Meng, A., Jia, S.
- Source
- Full text @ Nat. Commun.
|
sec14l3/SEC14L2 are expressed in endothelial cells and co-localized with VEGFR2. a Expression pattern of sec14l3 examined by WISH. Trunk vascular expression of sec14l3 is shown at 24 hpf and the vertical line denotes the equivalent position of transverse sections in embryos at 19 hpf and 25 hpf. High-resolution views of the boxed regions are shown at right panels. Adjacent tissues are denoted. NT, neural tube; NC, notochord; DA, dorsal aorta; PCV, posterior cardinal vein; VC, vascular cord. Scale bars, 100 μm. b The absence of sec14l3 expression in the trunk vascular system of etsrp mutant embryos at 26 hpf. High-resolution views of the boxed regions are shown at right panels. Scale bars, 100 μm. c Quantitative RT-PCR of sec14l3 mRNA in both GFP+ cells and GFP- cells from Tg(fli1a:EGFP)y1 transgenic embryos at 24 hpf. sec14l3 is highly enriched in GFP+ cells (blue bars) relative to negative cells (red bars), using β-actin as the internal control. kdrl/vegfr2 mRNA serves as a positive control for vascular endothelial cells. A two-tailed t-test was used for statistical analysis. *p < 0.05, the exact p-values in each figure are shown in the Source data file. d SEC14L2 is co-localized with VEGFR2 in HUVECs. Red and green represent anti-VEGFR2 antibody and anti-SEC14L2 antibody staining signal respectively; blue symbolizes DAPI stained nucleus. Regions in the boxes are enlarged in the right corner. Scale bars, 20 μm in the original pictures and 5 μm in the enlarged panelssec14l3/SEC14L2 are expressed in endothelial cells and co-localized with VEGFR2. a Expression pattern of sec14l3 examined by WISH. Trunk vascular expression of sec14l3 is shown at 24 hpf and the vertical line denotes the equivalent position of transverse sections in embryos at 19 hpf and 25 hpf. High-resolution views of the boxed regions are shown at right panels. Adjacent tissues are denoted. NT, neural tube; NC, notochord; DA, dorsal aorta; PCV, posterior cardinal vein; VC, vascular cord. Scale bars, 100 μm. b The absence of sec14l3 expression in the trunk vascular system of etsrp mutant embryos at 26 hpf. High-resolution views of the boxed regions are shown at right panels. Scale bars, 100 μm. c Quantitative RT-PCR of sec14l3 mRNA in both GFP+ cells and GFP- cells from Tg(fli1a:EGFP)y1 transgenic embryos at 24 hpf. sec14l3 is highly enriched in GFP+ cells (blue bars) relative to negative cells (red bars), using β-actin as the internal control. kdrl/vegfr2 mRNA serves as a positive control for vascular endothelial cells. A two-tailed t-test was used for statistical analysis. *p < 0.05, the exact p-values in each figure are shown in the Source data file. d SEC14L2 is co-localized with VEGFR2 in HUVECs. Red and green represent anti-VEGFR2 antibody and anti-SEC14L2 antibody staining signal respectively; blue symbolizes DAPI stained nucleus. Regions in the boxes are enlarged in the right corner. Scale bars, 20 μm in the original pictures and 5 μm in the enlarged panelsEXPRESSION / LABELING:
PHENOTYPE:
|
|
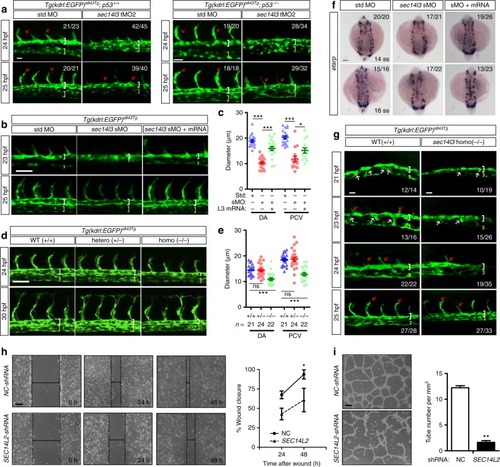
sec14l3/SEC14L2 are required for vascular formation in vivo and in vitro. asec14l3-tMO injection impairs arterial-venous segregation and luminal formation in zebrafish. Embryos of Tg(kdrl:EGFP)s843Tg;p53+/+ (left panel) or Tg(kdrl:EGFP)s843Tg;p53−/− fish (right panel) were used. White and yellow brackets indicate DA and PCV respectively; red arrows indicate ISVs sprouting. The ratio in the right corner indicates the number of embryos with observed pattern/the total number of observed embryos. b sec14l3-sMO mediated knockdown of sec14l3 causes trunk vascular defects. 0.5 ng sec14l3-sMO and 150 pg sec14l3 mRNA were co-injected for rescue experiment. c Statistical analyses of DA and PCV diameters at 25 hpf in b(n = 20 embryos). d Vasculature defects in sec14l3 mutant embryos. Heterozygous mutants were intercrossed and embryos were harvested for vasculature observation and genotyping analysis individually. e Statistical analyses of DA and PCV diameters at 30 hpf in d. The total number of embryos in each genotype is indicated. f sec14l3-sMO injection causes angioblast migration defects. 0.5 ng sec14l3-sMO and 100 pg sec14l3mRNA were co-injected for rescue experiment. WISH using etsrp probe marks the medial (arrowhead) and lateral (arrow) progenitor populations simultaneously. g Ventral sprouting defects in sec14l3 mutant embryos. hSEC14L2 knockdown inhibits the in vitro wound closure process. HUVECs infected with control or SEC14L2 shRNA were used for wound closure observation. The ratio of recovered wound width at a specific time point post-wounding to the initial wounding width was calculated. Dashed lines indicate wound edges. Statistical data are shown (n = 3). i SEC14L2knockdown inhibits in vitro tube formation. HUVECs were infected with shRNA-packed lentivirus and the complete tube number was scored per field at 24 h after plating. The statistical data are shown on the right (n = 3 independent experiments, 60 fields total). All statistical data are shown as mean ± SEM. One-way ANOVA tests were used for statistical analyses in c, e and t-tests were used for h–i. *p < 0.05; **p < 0.01; ***p < 0.01; ns, not significant. Scale bars, 25 μm for a, 50 μm for b, d, g, and h–i, 100 μm for f. Source data are provided as a Source Data file EXPRESSION / LABELING:
PHENOTYPE:
|
|
SEC14L2/sec14l3 knockdown attenuates VEGF signaling. a SEC14L2knockdown counteracts VEGFa-motivated p-ERK and p-AKT levels in HUVECs. HUVECs were infected with NC or SEC14L2 shRNA for 48 h. After VEGFa stimulation for 5, 15 or 30 min, cell lysates were harvested and immunoblotted with indicated antibodies. b, c Statistical results of relative p-ERK (b) and p-AKT (c) levels in a. The grey intensity of each band was measured for calculating the ratio of p-ERK to ERK (b) and p-AKT to AKT (c). Data are then normalized to control group with 0’ stimulation and represented as mean ± SEM from three independent experiments. d–eDepletion of sec14l3 decreases p-Erk (d) and p-Akt (e) levels in zebrafish embryos. Embryos from intercrossing sec14l3 heterzygous in Tg(kdrl: GFP)s843Tg transgenic background were harvested at 23 hpf for p-Erk antibody immunostaining (d) or at 25 hpf for p-Akt antibody staining (e). DA, dorsal aorta. The ratio in the right corner indicates the number of embryos with reduced staining/the number of observed embryos. Scale bars, 50 μm. f The defective DA/PCV lumen formation in sec14l3 morphants can be partially rescued by ca-MEK or ca-AKT mRNA injection. Five nanograms std-MO or sec14l3-tMO2 in combination with 50 or 100 pg ca-MEK or ca-AKT mRNA was injected into one-cell stage embryos. Their morphology (the upper panel) or vasculature defects (the bottom panel) at 25 hpf are separately shown. The ratio in the right corner indicates the number of embryos with indicated morphology/the number of observed embryos. Scale bars, 50 μm. g Statistic results of the DA (upper panel) or PCV (bottom panel) luminal diameters of each group at 30 hpf. Twenty embryos of each group from one representative experiment were calculated and shown here. To quantify the DA and PCV luminal diameter for an embryo, five different vessel regions of the same fish were measured to calculate the mean value, which was used to represent its vessel diameter of this fish. Three independent experiments were carried out. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant. ANOVA tests were used for statistical analyses. Source data are provided as a Source Data file EXPRESSION / LABELING:
PHENOTYPE:
|
|
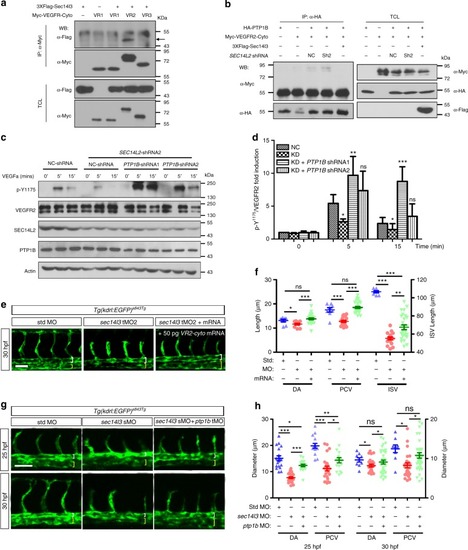
Enhanced VEGFR2-Y1175 phosphorylation restores SEC14L2/sec14l3depletion effects. a Sec14l3 specifically interacts with the cytoplasmic region of VEGFR2 in HEK293T cells. The cytoplasmic region of VEGFR1, VEGFR2 or VEGFR3 was individually transfected with Flag-tagged Sec14l3 into HEK293T cells for immunoprecipitation (IP) and immunoblotting. TCL, total cell lysate. b SEC14L2/Sec14l3 could alleviate the interaction between PTP1B and the VEGFR2-cyto in HEK293T cells. HA-tagged PTP1B and Myc-tagged VEGFR2-cyto were co-transfected into HEK293T cells with Flag-tagged Sec14l3 or SEC14L2 shRNA. After 72 hpf, cells were harvested and lysed for immunoprecipitation assay using an HA antibody. WB, western blot. c, d PTP1B knockdown rescues p-VEGFR2-Y1175 level inhibited by SEC14L2 knockdown in HEK293T cells. PTP1B shRNA1 or shRNA2 was transfected into SEC14L2 shRNA stable HEK293T cells with VEGFR2 overexpression. p-VEGFR2-Y1175 levels were checked after 100 ng ml−1 VEGFa stimulation for 0, 5 or 15 min. The loading volume was adjusted for the same amount of total VEGFR2 level in each lane. Quantification data of the relative p-VEGFR2-Y1175 level from three independent experiments are shown in d (n = 3). e The cytoplasmic region of VEGFR2 mRNA rescues luminal defects in sec14l3 morphants. Five nanograms std-MO or sec14l3-tMO2 in combination with 50 pg VEGFR2-cyto mRNA was injected into one-cell stage embryos from Tg(kdrl: GFP)s843Tg transgenic fish, and harvested at 30 hpf for the measurement of luminal diameters /ISV length. f Statistical result of the luminal diameters of DA, PCV, and ISV in e. 20 embryos of each group from one representative experiment were calculated and shown here. Three independent experiments were carried out. g, h ptp1b knockdown partially restores the lumen size of DA/PCV in zebrafish sec14l3 morphants. Tg(kdrl:GFP)s843Tgtransgenic embryos were injected with 0.5 ng std-MO or sec14l3-sMO in combination with 5 ng ptp1b-tMO and harvested at 25 and 30 hpf for the measurement of luminal diameters. Twenty embryos of each group were calculated, and statistical data from three independent experiments are shown in h. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant. ANOVA tests were used for statistical analyses in d, f,and h. Scale bars, 50 μm. Source data are provided as a Source Data file |
|
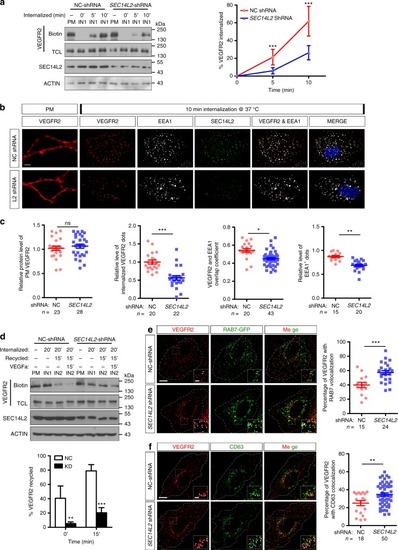
SEC14L2 knockdown disturbs VEGFR2 internalization and recycling processes. a Western blotting results show impairment of biotinylated VEGFR2 internalization in SEC14L2 knockdown cells. VEGFR2-overexpressing HEK293T cells were transfected with NC or SEC14L2shRNA for internalization assay described as supplementary Fig. 10. Quantified internalized VEGFR2 levels are shown on the right as mean ± SD from three independent experiments (n = 3). Red and blue lines represent control and SEC14L2 shRNA transfected group, respectively. TCL, total cell lysate. ***p < 0.001. b, c Analysis of VEGFR2 internalization based on immunostaining assay in HUVECs. HUVEC cells infected with NC or SEC14L2 shRNA were cultured for internalization assay and then cells were fixed for immunostaining with anti-EEA1 (white) and anti-SEC14L2 (green) antibodies. Quantification data of plasma membrane VEGFR2 (red) before internalization, internalized VEGFR2 after internalization, overlapping coefficient between internalized VEGFR2 and EEA1 (n = 40 cells) as well as EEA1 positive dots are shown individually in c. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant. d Western blot analysis of VEGFR2 recycling over a 35 min time course. VEGFR2-overexpressing HEK293T cells were transfected with NC- or SEC14L2 shRNA for 48 h and used for recycling assay as detailed in Supplementary Fig. 10. The recycling rate of VEGFR2 was measured with or without 100 ng ml−1 VEGFa stimulation. Quantification data are shown as mean ± SD from three independent experiments on the right (n = 3). **p < 0.01; ***p < 0.001. e, f SEC14L2knockdown promotes VEGFR2 localization in RAB7+ (e) or CD63+ (f) compartments. HUVEC cells infected with NC or SEC14L2 shRNA were transfected with RAB7-EGFP (e) or harvested directly (f) for anti-VEGFR2 (red) and EGFP/CD63 (green) staining. Regions in the boxes are enlarged in the right corner. Scale bars, 10 μm. The percentage of VEGFR2 co-localized with RAB7/CD63 is shown individually in the right, n indicates observed cell number. **p < 0.01; ***p < 0.001. Two-way ANOVA tests were used for a, d and t-tests for c, e, and f. Source data are provided as a Source Data file |