- Title
-
3D Visualization of Developmental Toxicity of 2,4,6-Trinitrotoluene in Zebrafish Embryogenesis Using Light-Sheet Microscopy
- Authors
- Eum, J., Kwak, J., Kim, H.J., Ki, S., Lee, K., Raslan, A.A., Park, O.K., Chowdhury, M.A., Her, S., Kee, Y., Kwon, S.H., Hwang, B.J.
- Source
- Full text @ Int. J. Mol. Sci.
|
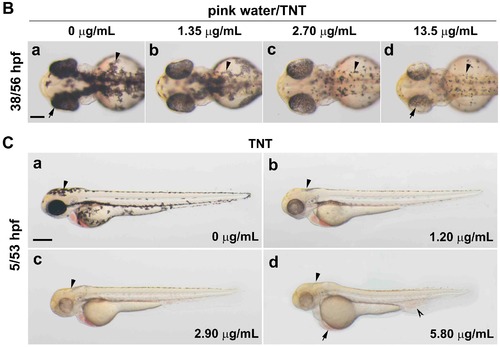
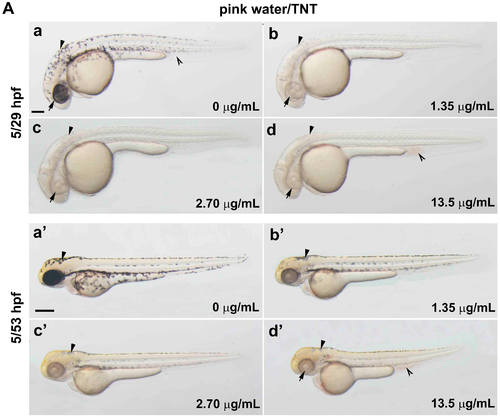
Trinitrotoluene (TNT) in pink water causes developmental defects in zebrafish. (A) Bright-field lateral view of embryos treated with pink water. Embryos were exposed to pink water containing TNT at 0 µg/mL (a,a′), 1.35 µg/mL (b,b′), 2.70 µg/mL (c,c′), and 13.5 µg/mL (d,d′) from 5 till 29 hpf (a–d, scale bar 250 µm) or 53 hpf (a′–d′, scale bar 500 µm) and imaged using a stereoscope. Defects in pigmentation were evident at 29 hpf (a–d, arrow for eye color, arrowhead for body pigment, 3.2× magnification) and at 53 hpf (a′–d′, arrow for abnormal heart, arrowhead for body pigment, 2.5× magnification). Morphological changes in body shape and a defect in blood circulation (split arrowhead) were observed in embryos treated with pink water containing 13.5 µg/mL of TNT at 53 hpf (d′). 5/29 hpf, treatment starting at 5 hpf, observation at 29 hpf; 5/53 hpf, treatment starting at 5 hpf, observation at 53 hpf; (B) Dorsal views of embryos treated with pink water from 38–56 hpf. (a–d) Embryos show a dose-dependent pigmentation defect. Less pigment is visible in the eye (arrow) and trunk melanocytes (arrowhead). 8× magnification, scale bar 250 µm. 38/56 hpf, treatment starting at 38 hpf, observation at 56 hpf; (C) Embryos treated with TNT at 0 µg/mL (a), 1.20 µg/mL (b), 2.90 µg/mL (c), and 5.80 µg/mL (d) from 5 hpf had similar phenotypes to embryos treated with pink water, including pigmentation defect (arrowhead), abnormal heart (arrow), and defective blood circulation (split arrowhead). 5/53 hpf, treatment starting at 5 hpf, observation at 53 hpf, scale bar 500 µm. |
|
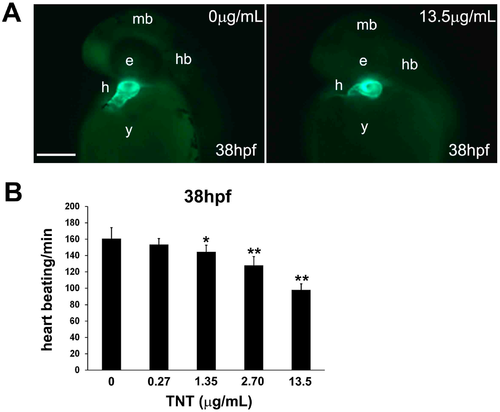
Trinitrotoluene (TNT) in pink water causes defects in heart development and function. (A) Tg(cmlc2:EGFP) embryos treated with pink water have myocardiac defects. Embryonic myocardiocytes were imaged at 38 hpf (hours post fertilization) with heads orientated upwards, scale bar 500 µm. Myocardiocytes in the atrium and ventricle were visualized by enhanced green fluorescent protein (EGFP) fluorescence in controls (a) and in embryos treated with pink water containing 13.5 µg/mL TNT for 33 h (b); (B) Heart rate is dose-dependently decreased in 38 hpf embryos by exposure to pink water containing TNT at 0, 0.27, 1.35, 2.70 and 13.5 µg/mL (n = 20 each). Following exposure to pink water from 5 hpf, Tg(cmlc2:EGFP) embryonic hearts were imaged laterally at 38 hpf using an Axioimager II fluorescence microscope (Carl Zeiss, Overkochen, Germany). * p < 0.05, ** p < 0.001. |
|
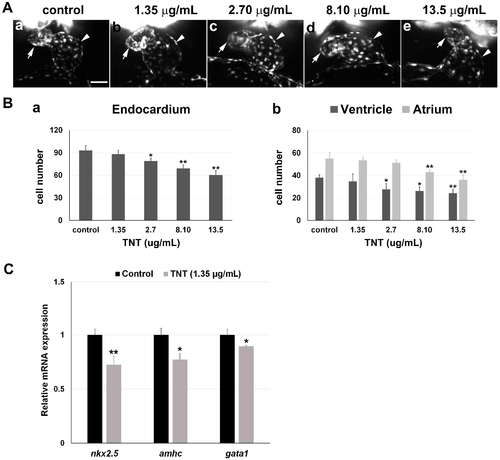
3D light-sheet imaging/SPIM (Single Plane Illumination Microscopy) of TNT cardiac toxicity. (A) TNT in pink water caused abnormal cardiac looping in a dose-dependent manner. (a–e) Endocardiums were visualized by SPIM in 38 hpf Tg(fli1a:EGFP) embryos treated with pink water containing TNT at 0 (a, control), 1.35 (b), 2.70 (c), 8.10 (d) and 13.5 µg/mL (e) from 5 hpf (2 experiments, n = 5 for each treatment). Live 3D reconstructions of the hearts were generated using Arivis software. Arrow, atrium; arrowhead, ventricle. 10× water lens, scale bar 50 µm. 3D reconstruction of each heart imaging is individually shown in Supplementary video 1A–E, respectfully; (B) (a) Treatment with pink water significantly reduced endocardial cell number in a dose-dependent manner; (b) The endocardium in the atrium was affected by TNT toxicity to a greater extent than the endocardium in the ventricle. * p < 0.05, ** p < 0.001; (C) Quantitative real time PCR (qPCR) analysis to measure mRNA expression of two heart-specific genes, nkx2.5 and amhc, and a blood specific gene, gata1, in the embryos treated with 0 and 1.35 µg/mL TNT from 5 till 36 hpf. Data are the mean ± SEM of three independent samples, differences between the means were evaluated with an independent-samples t-test. * p = 0.001, ** p < 0.0005. |
|
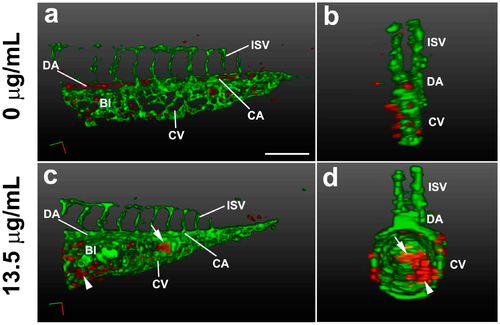
3D light-sheet/SPIM imaging of the TNT circulation defect. The vasculature (green) and blood cells (red) of the posterior trunk were visualized in Tg(gata1:DsRed/fli1a:EGFP) embryos at 38 hpf. 3D reconstructions are shown at lateral view (a,c) and transverse view (b,d), using Arivis software (surface function). (a,b) Embryos treated for 33 h with control E3 media; (c,d) Embryos treated for 33 h with pink water containing 13.5 µg/mL TNT showing swollen body structure with abnormal blood islands (arrowhead) and blood accumulation (arrow). 20X water lens, 0.8 zoom. Scale bar, 150 µm. BI, blood Island; CA, caudal artery; CV, caudal vein; DA, dorsal aorta; ISV, intersegmental vessel. The 3D movies of the reconstructions are presented in Supplementary video 2A,B. |
|
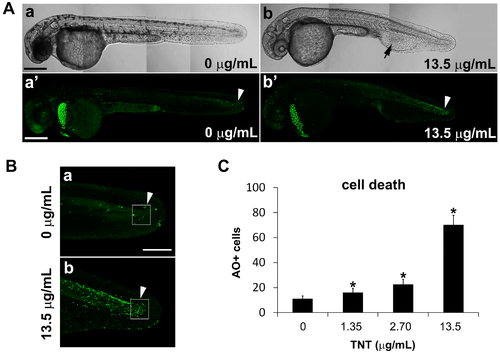
TNT in pink water increases the number of apoptotic cells in zebrafish embryos at 35 hpf. (A) Bright field images (a,b) and fluorescence images of acridine orange staining (a′,b′) by confocal microscopy show apoptosis in live whole embryos following treatment from 5–35 hpf with pink water containing TNT at 0 (a,a′) and 13.5 µg/mL (b,b′). Four tiled confocal images were combined to show the whole embryo in each case. Scale bar 500 µm. TNT treatment causes defective development of blood island and abnormal blood circulation (b, arrow) and more apoptosis in actively developing tissues of the tail (b′, arrowhead) compared with control (a′, arrowhead); (B) Magnified view of the tail regions of the embryos in (A) showing apoptotic cells (green dots); (a) is from A (a′) and (b) is from A (b′) respectfully. Scale bar 150 µm; (C) Number of acridine orange-positive apoptotic cells (AO + cells) in the boxed area in actively developing tail tissue (B, arrowhead) was counted at different concentrations of TNT using an Axioimager II fluorescence microscope. Cell death in TNT-treated embryos increased in a dose-dependent manner. n = 10 for each treatment, * p < 0.001. |